Abstract
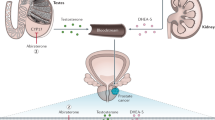
Prostate cancer is the most hormone sensitive of all cancers. However, any hormonal therapeutic strategy must take into account the fact that two almost equivalent sources of androgens act in the prostate, namely testosterone of testicular origin, and the locally produced androgens testosterone and dihydrotestosterone (DHT) derived from dehydroepiandrosterone of adrenal origin. Combined androgen blockade—medical or surgical castration plus a pure antiandrogen—would, therefore, be the logical first-line treatment for prostate cancer, although castration or an antiandrogen alone is still chosen in the majority of cases. Although long-term control, or even cure, is possible when combined androgen blockade is used when the tumor is localized, resistance to treatment invariably develops in patients when start of treatment is delayed until the disease has become metastatic. This observation can be explained either by elevated levels of the androgen receptor, which can increase the response to low levels of androgens and also modify the response to antiandrogens; or by local biosynthesis of androgens. Research to identify new and more potent antiandrogens, as well as blockers of peripheral and adrenal androgen biosynthesis—such as abiraterone—could be of great importance.
Key Points
-
Testicular androgens account for only 50–60% of total testosterone in the prostate, with the remainder produced by intraprostatic conversion of adrenal steroids
-
Prostate cancer is most frequently treated with medical or surgical castration alone, but this treatment does not ablate androgens of extratesticular origin, and castration resistance usually develops
-
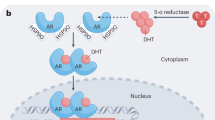
Castration resistance could be caused by increased levels of the androgen receptor or by intraprostatic synthesis of androgens from adrenal steroids or, possibly, from cholesterol
-
Combined androgen blockade with a pure antiandrogen in addition to surgical or medical castration seems, therefore, to be a scientifically sound approach to managing patients with prostate cancer
-
Novel agents such as abiraterone, which blocks androgen biosynthesis, have shown promise in patients with prostate cancer, especially when combined with glucocorticoid treatment
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
American Cancer Society. Cancer Facts & Figures 2010. American Cancer Society [online], (2010).
Lichtenberg, F. R. Measuring the health impacts of medical innovation and expenditure. Presented at the Health Services Research Seminar Series, University of Minnesota, Minnesota, USA, 2002.
Labrie, F. et al. Antifertility effects of LHRH agonists in the male. J. Androl. 1, 209–228 (1980).
Labrie, F. et al. Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr. Reviews 26, 361–379 (2005).
Labrie, F. et al. New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen. Clin. Invest. Med. 5, 267–275 (1982).
Labrie, F., Dupont, A. & Bélanger, A. Complete androgen blockade in the treatment oF prostate cancer. In Important Advances in Oncology (eds de Vita, V. T., Hellman, S. & Rosenberg, S. A.) 193–217 (J. B. Lippincott, Philadelphia, 1985).
Crawford, E. D. et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N. Engl. J. Med. 321, 419–424 (1989).
Labrie, F. Multiple intracrine hormonal targets in the prostate: opportunities and challenges. BJU Int. 100, 48–51 (2007).
Arnst, C. Developments to watch. Why did prostate cancer death rates fall? Business Week 92, 3853 (2003).
Peto, R. & Dalesio, O. Abstract 328. Presented at ECCO 12, Copenhagen, 2003.
Bélanger, B. et al. Comparison of residual C-19 steroids in plasma and prostatic tissue of human, rat and guinea pig after castration: unique importance of extratesticular androgens in men. J. Steroid Biochem. 32, 695–698 (1989).
Labrie, F. et al. Comparable amounts of sex steroids are made outside the gonads in men and women: strong lesson for hormone therapy of prostate and breast cancer. J. Steroid Biochem. Mol. Biol. 113, 52–56 (2009).
Labrie, F. Endocrinology and the prostate. Preface. Best Pract. Res. Clin. Endocrinol. Metab. 22, vii–ix (2008).
Labrie, F. Hormonal therapy in prostate cancer. In Neuroendocrinology, The Normal Neuroendocrine System, Progress in Brain Research (eds Martini, L., Chrousos, G. P., Labrie, F., Pacak, K. & Pfaff, D. E.) 321–341 (Elsevier, Oxford, 2010).
Labrie, F. Intracrinology. Mol. Cell. Endocrinol. 78, C113–C118 (1991).
Labrie, C. et al. Stimulation of androgen-dependent gene expression by the adrenal precursors dehydroepiandrosterone and androstenedione in the rat ventral prostate. Endocrinology 124, 2745–2754 (1989).
Labrie, F. et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J. Steroid Biochem. Mol. Biol. 99, 182–188 (2006).
Nishiyama, T., Hashimoto, Y. & Takahashi, K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin. Cancer Res. 10, 7121–7126 (2004).
Mostaghel, E. A. et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 67, 5033–5041 (2007).
Yoon, F. H. et al. Testosterone recovery after prolonged androgen suppression in patients with prostate cancer. J. Urol. 180, 1438–1444 (2008).
Labrie, C. et al. Stimulation of androgen-dependent gene expression by the adrenal precursors dehydroepiandrosterone and androstenedione in the rat ventral prostate. Endocrinology 124, 2745–2754 (1989).
Huggins, C. & Hodges, C. V. Studies of prostatic cancer. I. Effect of castration, estrogen and androgen injections on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 293–307 (1941).
Labrie, F., Candas, B., Gomez, J. L. & Cusan, L. Can combined androgen blockade provide long-term control or possible cure of localized prostate cancer? Urology 60, 115–119 (2002).
Akaza, H. et al. The case for androgen deprivation as primary therapy for early stage disease: results from J-CaP and CaPSURE. J. Urol. 176, S47–S49 (2006).
Homma, Y. et al. Endocrine therapy with or without radical prostatectomy for T1b-T3N0M0 prostate cancer. Int. J. Urol. 11, 218–224 (2004).
Egawa, M. et al. Retrospective study on stage B prostate cancer in the Hokuriku District, Japan. Int. J. Urol. 11, 304–309 (2004).
Ueno, S. et al. Efficacy of primary hormonal therapy for patients with localized and locally advanced prostate cancer: a retrospective multicenter study. Int. J. Urol. 13, 1494–1500 (2006).
Akaza, H. et al. Superior anti-tumor efficacy of bicalutamide 80 mg in combination with a luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist monotherapy as first-line treatment for advanced prostate cancer: interim results of a randomized study in Japanese patients. Jpn J. Clin. Oncol. 34, 20–28 (2004).
Akaza, H. Trends in primary androgen depletion therapy for patients with localized and locally advanced prostate cancer: Japanese perspective. Cancer Sci. 97, 243–247 (2006).
Dupont, A. et al. Combination therapy with flutamide and [D-Trp6]LHRH ethylamide for stage C prostatic carcinoma. Eur. J. Cancer Clin. Oncol. 24, 659–666 (1988).
Dupont, A. et al. Combination therapy with Flutamide and the LHRH agonist [D-Trp6-des-Gly-NH2]LHRH ethylamide in stage C prostatic carcinoma. Br. J. Urol. 72, 629–634 (1993).
Cooperberg, M. R., Grossfeld, G. D., Lubeck, D. P. & Carroll, P. R. National practice patterns and time trends in androgen ablation for localized prostate cancer. J. Natl Cancer Inst. 95, 981–989 (2003).
Denis, L. J. et al. Maximal androgen blockade: final analysis of EORTC Phase III trial 30853. Eur. Urol. 33, 144–151 (1998).
Caubet, J. F. et al. Maximum androgen blockade in advanced prostate cancer: a meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens. Urology 49, 71–78 (1997).
Bennett, C. L. et al. Maximum androgen-blockade with medical or surgical castration in advanced prostate cancer: a meta-analysis of nine published randomized controlled trials and 4128 patients using Flutamide. Prostate Cancer Prostatic Dis. 2, 4–8 (1999).
Prostate Cancer Triallists' Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 355, 1491–1498 (2000).
Eisenberger, M. A. et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N. Engl. J. Med. 339, 1036–1042 (1998).
Labrie, F. et al. Benefits of combination therapy with Flutamide in patients relapsing after castration. Br. J. Urol. 61, 341–346 (1988).
Levine, G. N. et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation 121, 833–840 (2010).
Akaza, H. et al. Efficacy of primary hormone therapy for localized or locally advanced prostate cancer: results of a 10-year follow-up. BJU Int. 98, 573–579 (2006).
Akaza, H. Current status and prospects of androgen depletion therapy for prostate cancer. Best Pract. Res. Clin. Endocrinol. Metab. 22, 293–302 (2008).
Namiki, M., Kitagawa, Y., Mizokami, A. & Koh, E. Primary combined androgen blockade in localized disease and its mechanism. Best Pract. Res. Clin. Endocrinol. Metab. 22, 303–315 (2008).
Labrie, F. & Veilleux, R. A wide range of sensitivities to androgens develops in cloned Shionogi mouse mammary tumor cells. Prostate 8, 293–300 (1986).
Fowler, J. E. Jr & Whitmore, W. F. Jr. The response of metastatic adenocarcinoma of the prostate to exogenous testosterone. J. Urol. 126, 372–375 (1981).
Maddy, J. A., Winternitz, W. W. & Norrell, H. Cryohypophysectomy in the management of advanced prostatic cancer. Cancer 28, 322–328 (1971).
Drago, J. R. et al. Clinical effect of aminoglutethimide, medical adrenalectomy, in treatment of 43 patients with advanced prostatic carcinoma. Cancer 53, 1447–1450 (1984).
Murray, R. & Pitt, P. Treatment of advanced prostatic cancer, resistant to conventional therapy, with aminoglutethimide. Eur. J. Cancer Clin. Oncol. 21, 453–458 (1985).
Janknegt, R. A. et al. Orchiectomy and Nilutamide or placebo as treatment of metastatic prostatic cancer in a multinational double-blind randomized trial. J. Urol. 149, 77–83 (1993).
Collinson, M. P., Daniel, F., Tyrrell, C. J. & Teasdale, C. Response of carcinoma of the prostate to withdrawal of flutamide. Br. J. Urol. 72, 662–663 (1993).
Herrada, J., Dieringer, P. & Logothetis, C. J. Characterization of patients with androgen-independent prostatic carcinoma whose serum prostate specific antigen decreased following flutamide withdrawal. J. Urol. 155, 620–623 (1996).
Kelly, W. K. & Scher, H. I. Prostate specific antigen decline after antiandrogen withdrawal: the flutamide withdrawal syndrome. J. Urol. 149, 607–609 (1993).
Kelly, W. K., Slovin, S. & Scher, H. I. Steroid hormone withdrawal syndromes. Pathophysiology and clinical significance. Urol. Clin. North Am. 24, 421–431 (1997).
Labrie, F., Veilleux, R. & Fournier, A. Low androgen levels induce the development of androgen-hypersensitive cells clones in Shionogi mouse mammary carcinoma cells in culture. J. Natl Cancer Inst. 80, 1138–1147 (1988).
Chen, C. D. et al. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 10, 33–39 (2004).
Scher, H. I. & Sawyers, C. L. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J. Clin. Oncol. 23, 8253–8261 (2005).
Mulders, P. F. & Schalken, J. A. Measuring therapeutic efficacy in the changing paradigm of castrate-resistant prostate cancer. Prostate Cancer Prostatic Dis. 12, 241–246 (2009).
McPhaul, M. J. Mechanisms of prostate cancer progression to androgen independence. Best Pract. Res. Clin. Endocrinol. Metab. 22, 373–388 (2008).
Harris, W. P., Mostaghel, E. A., Nelson, P. S. & Montgomery, B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 6, 76–85 (2009).
Mostaghel, E. A., Montgomery, B. & Nelson, P. S. Castration-resistant prostate cancer: targeting androgen metabolic pathways in recurrent disease. Urol. Oncol. 27, 251–257 (2009).
Chen, S. et al. New experimental models for aromatase inhibitor resistance. J. Steroid Biochem. Mol. Biol. 106, 8–15 (2007).
Koivisto, P. et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 57, 314–319 (1997).
Visakorpi, T. et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet. 9, 401–406 (1995).
Culig, Z. et al. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol. Endocrinol. 7, 1541–1550 (1993).
Taplin, M. E. et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N. Engl. J. Med. 332, 1393–1398 (1996).
Gregory, C. W., Johnson, R. T. Jr, Mohler, J. L., French, F. S. & Wilson, E. M. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 61, 2892–2898 (2001).
Mizokami, A. et al. The adrenal androgen androstenediol is present in prostate cancer tissue after androgen deprivation therapy and activates mutated androgen receptor. Cancer Res. 64, 765–771 (2004).
Takeda, H. et al. Androgen receptor content of prostate carcinoma cells estimated by immunohistochemistry is related to prognosis of patients with stage D2 prostate carcinoma. Cancer 77, 934–940 (1996).
Taplin, M. E. & Balk, S. P. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J. Cell. Biochem. 91, 483–490 (2004).
Labrie, F. et al. Pure selective estrogen inhibitors—new molecules having absolute cell specificity ranging from pure antiestrogens to complete estrogen-like activities. In Advances in Protein Chemistry: Drug Discovery and Design (ed. Scolnick, E. M.) 293–368 (Academic Press, San Diego, CA, 2001).
Barbier, O. & Bélanger, A. Inactivation of androgens by UDP-glucuronosyltransferases in the human prostate. Best Pract. Res. Clin. Endocrinol. Metab. 22, 259–270 (2008).
Labrie, C., Bélanger, A. & Labrie, F. Androgenic activity of dehydroepiandrosterone and androstenedione in the rat ventral prostate. Endocrinology 123, 1412–1417 (1988).
Pelletier, G. Expression of steroidogenic enzymes and sex-steroid receptors in human prostate. Best Pract. Res. Clin. Endocrinol. Metab. 22, 223–228 (2008).
El-Alfy, M. et al. Localization of type 5 17β-hydroxysteroid dehydrogenase, 3 β-hydroxysteroid dehydrogenase and androgen receptor in the human prostate by in situ hybridization and immunocytochemistry. Endocrinology 140, 1481–1491 (1999).
Nakamura, Y. et al. In situ androgen producing enzymes in human prostate cancer. Endocr. Relat. Cancer 12, 101–107 (2005).
Luu-The, V., Bélanger, A. & Labrie, F. Androgen biosynthetic pathways in the human prostate. Best Pract. Res. Clin. Endocrinol. Metab. 22, 207–221 (2008).
Evaul, K., Li, R., Papari-Zareei, M., Auchus, R. J. & Sharifi, N. 3Beta-hydroxysteroid dehydrogenase is a possible pharmacological target in the treatment of castration-resistant prostate cancer. Endocrinology 151, 3514–3520 (2010).
Mizokami, A. et al. Prostate cancer stromal cells and LNCaP cells coordinately activate the androgen receptor through synthesis of testosterone and dihydrotestosterone from dehydroepiandrosterone. Endocr. Relat. Cancer 16, 1139–1155 (2009).
Stanbrough, M. et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 66, 2815–2825 (2006).
Nishiyama, T., Ikarashi, T., Hashimoto, Y., Wako, K. & Takahashi, K. The change in the dihydrotestosterone level in the prostate before and after androgen deprivation therapy in connection with prostate cancer aggressiveness using the Gleason score. J. Urol. 178, 1282–1288; discussion 1288–1289 (2007).
Agus, D. B. et al. Prostate cancer cell cycle regulators: response to androgen withdrawal and development of androgen independence. J. Natl Cancer Inst. 91, 1869–1876 (1999).
Rosner, J. M., Macome, J. C. & Cardinali, D. P. In vitro biosynthesis of sterols and steroids by rat submaxillary glands. Endocrinology 85, 1000–1003 (1969).
Voutilainen, R. & Miller, W. L. Developmental expression of genes for the stereoidogenic enzymes P450scc (20, 22-desmolase), P450c17 (17 alpha-hydroxylase/17, 20-lyase), and P450c21 (21-hydroxylase) in the human fetus. J. Clin. Endocrinol. Metab. 63, 1145–1150 (1986).
Labrie, F., Simard, J., Singh, S. M. & Candas, B. Estimated potency of Casodex: a problematic design. Urology 50, 309–313 (1997).
Labrie, F., Simard, J., Coquet, A., Leblanc, G. & Candas, B. Reply by the authors. Urology 54, 195–197 (1999).
Simard, J., Singh, S. M. & Labrie, F. Comparison of in vitro effects of the pure antiandrogens OH-Flutamide, Casodex and Nilutamide on androgen-sensitive parameters. Urology 49, 580–589 (1997).
Luo, S. et al. Relative potencies of Flutamide and Casodex: preclinical studies. Endocr. Relat. Cancer 3, 229–241 (1996).
Labrie, F. et al. New concepts on the androgen sensitivity of prostate cancer. In Progress in Clinical and Biological Research. Prostate Cancer Part A: Research. Endocrine Treatment and Histopathology (eds Murphy, G. P., Khoury, S., Kuss, R., Chatelain, C. & Denis, L.) 145–172 (Liss, New York, 1987).
Debes, J. D. & Tindall, D. J. Mechanisms of androgen-refractory prostate cancer. N. Engl. J. Med. 351, 1488–1490 (2004).
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009).
Scher, H. I. et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375, 1437–1446 (2010).
Reid, A. H. et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J. Clin. Oncol. 28, 1489–1495 (2010).
Attard, G. et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 (2008).
Attard, G. et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 27, 3742–3748 (2009).
O'Donnell, A. et al. Hormonal impact of the 17alpha-hydroxylase/C(17, 20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br. J. Cancer 90, 2317–2325 (2004).
Attard, G. Abiraterone clinical data. Presented at the 14th International Congress on Hormonal Steroids and Hormones & Cancer, Edinburgh, 2010.
Small, E. J. et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 24, 3089–3094 (2006).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares that he is the Director of Endorecherche Inc.
Rights and permissions
About this article
Cite this article
Labrie, F. Blockade of testicular and adrenal androgens in prostate cancer treatment. Nat Rev Urol 8, 73–80 (2011). https://doi.org/10.1038/nrurol.2010.231
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2010.231
This article is cited by
-
Preclinical models of prostate cancer — modelling androgen dependency and castration resistance in vitro, ex vivo and in vivo
Nature Reviews Urology (2023)
-
Anti-Müllerian hormone: a novel biomarker for aggressive prostate cancer? Emerging evidence from a prospective study of radical prostatectomies
Hormones (2023)
-
Advances in stem cell research for the treatment of primary hypogonadism
Nature Reviews Urology (2021)
-
A Time Scales Approach for Modeling Intermittent Hormone Therapy for Prostate Cancer
Bulletin of Mathematical Biology (2020)
-
Dehydroepiandrosterone Induces Temozolomide Resistance Through Modulating Phosphorylation and Acetylation of Sp1 in Glioblastoma
Molecular Neurobiology (2019)