Abstract
Stem cells have the ability to both differentiate into other mature cell types and maintain an undifferentiated state by self-renewal. These unique properties form the basis for stem cell use in organ replacement and tissue regeneration in clinical medicine. Currently, embryonic stem cells are the best-studied stem cell type. However alternative stem cells such as induced pluripotent stem cells and other adult stem cells are also being actively investigated for their potential for cell-based therapy. Among adult stem cells, emerging research has focused on evaluating the pluripotency potential of testis stem cells. To date, stem cells with embryonic-like potential have been created from adult testis germ cells. These cells could provide patient-specific, non-embryo-derived stem cells for men in the future.
Key Points
-
A need exists for pluripotent stem cells other than human embryonic cells for cell-based therapy owing to difficulties surrounding procurement, tissue rejection and tumorigenesis with these cells
-
Adult stem cells might provide an alternative source of multipotent stem cells with less rejection and decreased malignant potential than embryonic stem cells
-
Testicular stem cells with multipotent and even pluripotent potential have been isolated in mouse models and, more recently, in humans
-
To achieve the goal of cell-based therapy using any type of stem cell, issues of reliability and safety, in addition to efficiency and expandability, must be overcome
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007).
Park, I. H. et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451, 141–146 (2008).
Zhou, H. et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4, 381–384 (2009).
Kim, D. et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4, 472–476 (2009).
Greely, H. T. Moving human embryonic stem cells from legislature to lab: remaining legal and ethical questions. PLoS Med. 3, e143 (2006).
Scott, C. T. & Reijo Pera, R. A. The road to pluripotence: the research response to the embryonic stem cell debate. Hum. Mol. Genet. 17, R3–R9 (2008).
Hyun, I., Hochedlinger, K., Jaenisch, R. & Yamanaka, S. New advances in iPS cell research do not obviate the need for human embryonic stem cells. Cell Stem Cell 1, 367–368 (2007).
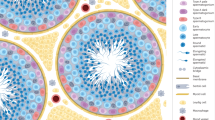
Amann, R. P. & Howards, S. S. Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J. Urol. 124, 211–215 (1980).
Clermont, Y. The cycle of the seminiferous epithelium in man. Am. J. Anat. 112, 35–51 (1963).
Heller, C. H. & Clermont, Y. Kinetics of the germinal epithelium in man. Recent Prog. Horm. Res. 20, 545–575 (1964).
Witschi, E. Migration of the germ cells of human embryos from the yolk sac to the primitive gonadal fold. Carnegie Institute Wash. Contrib. Embryol. 209, 67–80 (1948).
Gondos, B. & Hobel, C. J. Ultrastructure of germ cell development in the human fetal testis. Z. Zellforsch. Mikrosk. Anat. 119, 1–20 (1971).
Ezeh, U. I., Turek, P. J., Reijo, R. A. & Clark, A. T. Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer 104, 2255–2265 (2005).
Andrews, P. W. Teratocarcinomas and human embryology: pluripotent human EC cell lines. Review article. APMIS 106, 158–168 (1998).
Shamblott, M. J. et al. Derivation of pluirpotent stem cells from cultured human primordial germ cells. Proc. Natl Acad. Sci. USA 95, 13726–13731 (1998).
Oatley, J. M. & Brinster, R. L. Regulation of spermatogonial stem cell self-renewal in mammals. Annu. Rev. Cell Dev. Biol. 24, 263–286 (2008).
Dym, M. Spermatogonial stem cells of the testis. Proc. Natl Acad. Sci. USA 91, 11287–11289 (1994).
Yoshinaga, K. et al. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development 113, 689–699 (1991).
He, Z., Kokkinaki, M., Jiang, J., Dobrinski, I. & Dym, M. Isolation, characterization, and culture of human spermatogonia. Biol. Reprod. doi:10.1095/biolreprod.109.078550
Kristensen, D. M. et al. Presumed pluripotency markers UTF-1 and REX-1 are expressed in human adult testes and germ cell neoplasms. Hum. Reprod. 23, 775–782 (2008).
Clermont, Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 52, 198–236 (1972).
Ewing, L. L., Davis, J. C. & Zirkin, B. R. Regulation of testicular function: a spatial and temporal view. Int. Rev. Physiol. 22, 41–115 (1980).
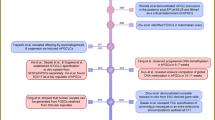
Kanatsu-Shinohara, M. et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell 119, 1001–1012 (2004).
Kanatsu-Shinohara, M. et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 69, 612–616 (2003).
Guan, K. et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440, 1199–1203 (2006).
Ko, K. et al. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell 5, 87–96 (2009).
Conrad, S. et al. Generation of pluripotent stem cells from adult human testis. Nature 456, 344–349 (2008).
Kossack, N. et al. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells 27, 138–149 (2009).
Golestaneh, N. et al. Pluripotent stem cells derived from adult human testes. Stem Cells Dev. 18, 1115–1126 (2009).
Mizrak, S. C. et al. Embryonic stem cell-like cells derived from adult human testis. Hum. Reprod. doi: 10.1093/humrep/dep354
Krizhanovsky, V. & Lowe, S. W. Stem cells: the promises and perils of p53. Nature 460, 1085–1086 (2009).
Kee, K., Angeles, V. T., Flores, M., Nguyen, H. N. & Reijo Pera, R. A. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature 462, 222–225 (2009).
Johnson, J., Canning, J., Kaneko, T., Pru, J. K. & Tilly, J. L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428, 145–150 (2004).
Johnson, J. et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell 122, 303–315 (2005).
Telfer, E. E. et al. On regenerating the ovary and generating controversy. Cell 122, 821–822 (2005).
Eggan, K., Jurga, S., Gosden, R., Min, I. M. & Wagers, A. J. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature 441, 1109–1114 (2006).
Thomson, J. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
P. J. Turek and R. A. Reijo Pera declare that they hold, or have applied for, patents related to testicular germline stem cells, on behalf of The Turek Clinic, San Francisco and Stanford University, CA, USA. K. Kee declares no competing interests.
Rights and permissions
About this article
Cite this article
Kee, K., Reijo Pera, R. & Turek, P. Testicular germline stem cells. Nat Rev Urol 7, 94–100 (2010). https://doi.org/10.1038/nrurol.2009.263
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrurol.2009.263
This article is cited by
-
Previously claimed male germline stem cells from porcine testis are actually progenitor Leydig cells
Stem Cell Research & Therapy (2018)
-
Amniotic fluid stem cell-based models to study the effects of gene mutations and toxicants on male germ cell formation
Asian Journal of Andrology (2012)
-
Reconstituting mammalian spermatogenesis using stem cells
Asian Journal of Andrology (2011)