Key Points
-
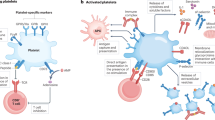
Platelets contain and can synthesize molecules capable of modulating adaptive and innate immune responses.
-
In systemic lupus erythematosus (SLE), platelets are activated, their abundance often reflects disease activity, and treatments for SLE can affect platelet functions.
-
Platelets express immune receptors, including FcγRIIA and Toll-like receptors, that could initiate platelet activation in SLE.
-
Complement activation during the course of SLE implicates platelets and supports a role for platelets in thrombosis in SLE.
-
Molecules such as CD40 ligand, IL-1, serotonin and danger-associated molecular patterns are present in platelets and can participate in the pathogenesis of SLE.
-
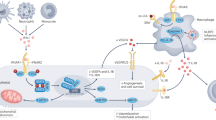
Platelets can shed membrane vesicles, known as microparticles or microvesicles, that convey inflammatory mediators and disseminate mitochondrial antigens for the formation of immune complexes.
Abstract
Dysregulation of lymphocyte function, accumulation of autoantibodies and defective clearance of circulating immune complexes and apoptotic cells are hallmarks of systemic lupus erythematosus (SLE). Moreover, it is now evident that an intricate interplay between the adaptive and innate immune systems contributes to the pathogenesis of SLE, ultimately resulting in chronic inflammation and organ damage. Platelets circulate in the blood and are chiefly recognized for their role in the prevention of bleeding and promotion of haemostasis; however, accumulating evidence points to a role for platelets in both adaptive and innate immunity. Through a broad repertoire of receptors, platelets respond promptly to immune complexes, complement and damage-associated molecular patterns, and represent a major reservoir of immunomodulatory molecules in the circulation. Furthermore, evidence suggests that platelets are activated in patients with SLE, and that they could contribute to the circulatory autoantigenic load through the release of microparticles and mitochondrial antigens. Herein, we highlight how platelets contribute to the immune response and review evidence implicating platelets in the pathogenesis of SLE.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lovgren, T., Eloranta, M. L., Bave, U., Alm, G. V. & Ronnblom, L. Induction of interferon-α production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50, 1861–1872 (2004).
Bengtsson, A. A. & Ronnblom, L. Systemic lupus erythematosus: still a challenge for physicians. J. Intern. Med. 281, 52–64 (2017).
Mahajan, A., Herrmann, M. & Munoz, L. E. Clearance deficiency and cell death pathways: a model for the pathogenesis of SLE. Front. Immunol. 7, 35 (2016).
Sisirak, V. et al. Digestion of chromatin in apoptotic cell microparticles prevents autoimmunity. Cell 166, 88–101 (2016).
Sjoberg, A. P., Trouw, L. A. & Blom, A. M. Complement activation and inhibition: a delicate balance. Trends Immunol. 30, 83–90 (2009).
Truedsson, L., Bengtsson, A. A. & Sturfelt, G. Complement deficiencies and systemic lupus erythematosus. Autoimmunity 40, 560–566 (2007).
Pickering, M. C., Botto, M., Taylor, P. R., Lachmann, P. J. & Walport, M. J. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv. Immunol. 76, 227–324 (2000).
Manderson, A. P., Botto, M. & Walport, M. J. The role of complement in the development of systemic lupus erythematosus. Annu. Rev. Immunol. 22, 431–456 (2004).
Esdaile, J. M. et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 44, 2331–2337 (2001).
Manzi, S. et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am. J. Epidemiol. 145, 408–415 (1997).
Bengtsson, C., Ohman, M. L., Nived, O. & Rantapaa Dahlqvist, S. Cardiovascular event in systemic lupus erythematosus in northern Sweden: incidence and predictors in a 7-year follow-up study. Lupus 21, 452–459 (2012).
Willis, R., Harris, E. N. & Pierangeli, S. S. Pathogenesis of the antiphospholipid syndrome. Semin. Thromb. Hemost 38, 305–321 (2012).
Chighizola, C. B., Raschi, E., Borghi, M. O. & Meroni, P. L. Update on the pathogenesis and treatment of the antiphospholipid syndrome. Curr. Opin. Rheumatol 27, 476–482 (2015).
Kapur, R., Zufferey, A., Boilard, E. & Semple, J. W. Nouvelle cuisine: platelets served with inflammation. J. Immunol. 194, 5579–5587 (2015).
Semple, J. W., Italiano, J. E. & Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 11, 264–274 (2011).
Grozovsky, R., Giannini, S., Falet, H. & Hoffmeister, K. M. Regulating billions of blood platelets: glycans and beyond. Blood 126, 1877–1884 (2015).
Machlus, K. R. & Italiano, J. E. Jr. The incredible journey: from megakaryocyte development to platelet formation. J. Cell Biol. 201, 785–796 (2013).
Lefrancais, E. et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 544, 105–109 (2017).
Fuentes, R. et al. Infusion of mature megakaryocytes into mice yields functional platelets. J. Clin. Invest. 120, 3917–3922 (2010).
Nieswandt, B., Varga-Szabo, D. & Elvers, M. Integrins in platelet activation. J. Thromb. Haemost. 7 (Suppl. 1), 206–209 (2009).
Clemetson, K. J. Platelets and primary haemostasis. Thromb. Res. 129, 220–224 (2012).
Boulaftali, Y., Hess, P. R., Kahn, M. L. & Bergmeier, W. Platelet immunoreceptor tyrosine-based activation motif (ITAM) signaling and vascular integrity. Circ. Res. 114, 1174–1184 (2014).
Karas, S. P., Rosse, W. F. & Kurlander, R. J. Characterization of the IgG-Fc receptor on human platelets. Blood 60, 1277–1282 (1982).
Gros, A. et al. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex-mediated inflammation in mice. Blood 126, 1017–1026 (2015).
King, S. M. & Reed, G. L. Development of platelet secretory granules. Semin. Cell Dev. Biol. 13, 293–302 (2002).
Maynard, D. M., Heijnen, H. F., Horne, M. K., White, J. G. & Gahl, W. A. Proteomic analysis of platelet α-granules using mass spectrometry. J. Thromb. Haemost. 5, 1945–1955 (2007).
Nieswandt, B., Pleines, I. & Bender, M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J. Thromb. Haemost. 9 (Suppl. 1), 92–104 (2011).
George, J. N. Platelets. Lancet 355, 1531–1539 (2000).
Cox, D., Kerrigan, S. W. & Watson, S. P. Platelets and the innate immune system: mechanisms of bacterial-induced platelet activation. J. Thromb. Haemost. 9, 1097–1107 (2011).
Zahn, A., Jennings, N., Ouwehand, W. H. & Allain, J. P. Hepatitis C virus interacts with human platelet glycoprotein VI. J. Gen. Virol. 87, 2243–2251 (2006).
Chaipan, C. et al. DC-SIGN and CLEC-2 mediate human immunodeficiency virus type 1 capture by platelets. J. Virol. 80, 8951–8960 (2006).
Rondina, M. T., Weyrich, A. S. & Zimmerman, G. A. Platelets as cellular effectors of inflammation in vascular diseases. Circ. Res. 112, 1506–1519 (2013).
Sreeramkumar, V. et al. Neutrophils scan for activated platelets to initiate inflammation. Science 346, 1234–1238 (2014).
Zhi, H. et al. Platelet activation and thrombus formation over IgG immune complexes requires integrin αIIbβ3 and Lyn kinase. PLoS ONE 10, e0135738 (2015).
Cloutier, N. et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc. Natl Acad. Sci. USA 115, E1550–E1559 (2018).
Boilard, E. et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327, 580–583 (2010).
Hitchcock, J. R. et al. Inflammation drives thrombosis after Salmonella infection via CLEC-2 on platelets. J. Clin. Invest. 125, 4429–4446 (2015).
Lax, S. et al. Platelet CLEC-2 protects against lung injury via effects of its ligand podoplanin on inflammatory alveolar macrophages in the mouse. Am. J. Physiol. Lung Cell Mol. Physiol. 313, L1016–L1029 (2017).
Nylander, A. N. et al. Podoplanin is a negative regulator of Th17 inflammation. JCI Insight 2, e92321 (2017).
Bruhns, P. & Jonsson, F. Mouse and human FcR effector functions. Immunol. Rev. 268, 25–51 (2015).
McKenzie, S. E. et al. The role of the human Fc receptor FcγRIIA in the immune clearance of platelets: a transgenic mouse model. J. Immunol. 162, 4311–4318 (1999).
Aslam, R. et al. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-α production in vivo. Blood 107, 637–641 (2006).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Thon, J. N. et al. T granules in human platelets function in TLR9 organization and signaling. J. Cell Biol. 198, 561–574 (2012).
Cognasse, F. et al. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br. J. Haematol. 141, 84–91 (2008).
Morrell, C. N., Aggrey, A. A., Chapman, L. M. & Modjeski, K. L. Emerging roles for platelets as immune and inflammatory cells. Blood 123, 2759–2767 (2014).
Speth, C. et al. Complement and platelets: Mutual interference in the immune network. Mol. Immunol. 67, 108–118 (2015).
Zufferey, A. et al. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood Adv. 1, 1773–1785 (2017).
Chapman, L. M. et al. Platelets present antigen in the context of MHC class I. J. Immunol. 189, 916–923 (2012).
Assoian, R. K., Komoriya, A., Meyers, C. A., Miller, D. M. & Sporn, M. B. Transforming growth factor-β in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 258, 7155–7160 (1983).
Petersen, F., Bock, L., Flad, H. D. & Brandt, E. Platelet factor 4-induced neutrophil-endothelial cell interaction: involvement of mechanisms and functional consequences different from those elicited by interleukin-8. Blood 94, 4020–4028 (1999).
Shi, G. et al. Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection. J. Clin. Invest. 124, 543–552 (2014).
Italiano, J. E. Jr & Battinelli, E. M. Selective sorting of α-granule proteins. J. Thromb. Haemost. 7 (Suppl. 1), 173–176 (2009).
Pignatelli, P. et al. Tumor necrosis factor-α as trigger of platelet activation in patients with heart failure. Blood 106, 1992–1994 (2005).
Negrotto, S. et al. Expression and functionality of type I interferon receptor in the megakaryocytic lineage. J. Thromb. Haemost. 9, 2477–2485 (2011).
Beaulieu, L. M. et al. Interleukin 1 receptor 1 and interleukin 1β regulate megakaryocyte maturation, platelet activation, and transcript profile during inflammation in mice and humans. Arterioscler. Thromb. Vasc. Biol. 34, 552–564 (2014).
Bester, J. & Pretorius, E. Effects of IL-1β, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci. Rep. 6, 32188 (2016).
Hamad, O. A. et al. Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J. Thromb. Haemost. 6, 1413–1421 (2008).
Denis, M. M. et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell 122, 379–391 (2005).
Hawrylowicz, C. M., Howells, G. L. & Feldmann, M. Platelet-derived interleukin 1 induces human endothelial adhesion molecule expression and cytokine production. J. Exp. Med. 174, 785–790 (1991).
Brown, G. T. & McIntyre, T. M. Lipopolysaccharide signaling without a nucleus: kinase cascades stimulate platelet shedding of proinflammatory IL-1β-rich microparticles. J. Immunol. 186, 5489–5496 (2011).
Landry, P. et al. Existence of a microRNA pathway in anucleate platelets. Nat. Struct. Mol. Biol. 16, 961–966 (2009).
McManus, D. D. & Freedman, J. E. MicroRNAs in platelet function and cardiovascular disease. Nat. Rev. Cardiol. 12, 711–717 (2015).
Laffont, B. et al. Activated platelets can deliver mRNA regulatory Ago2·microRNA complexes to endothelial cells via microparticles. Blood 122, 253–261 (2013).
Laffont, B. et al. Platelet microparticles reprogram macrophage gene expression and function. Thromb. Haemost. 115, 311–323 (2016).
Duchez, A. C. et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc. Natl Acad. Sci. USA 112, E3564–E3573 (2015).
Michael, J. V. et al. Platelet microparticles infiltrating solid tumors transfer miRNAs that suppress tumor growth. Blood 130, 567–580 (2017).
Boilard, E., Blanco, P. & Nigrovic, P. A. Platelets: active players in the pathogenesis of arthritis and SLE. Nat. Rev. Rheumatol. 8, 534–542 (2012).
Ferro, D. et al. Determinants of enhanced thromboxane biosynthesis in patients with systemic lupus erythematosus. Arthritis Rheum. 42, 2689–2697 (1999).
Nagahama, M. et al. Platelet activation markers and soluble adhesion molecules in patients with systemic lupus erythematosus. Autoimmunity 33, 85–94 (2001).
Tam, L. S. et al. Patients with systemic lupus erythematosus show increased platelet activation and endothelial dysfunction induced by acute hyperhomocysteinemia. J. Rheumatol. 30, 1479–1484 (2003).
Ekdahl, K. N. et al. Thrombotic disease in systemic lupus erythematosus is associated with a maintained systemic platelet activation. Br. J. Haematol. 125, 74–78 (2004).
Nhek, S. et al. Activated platelets induce endothelial cell activation via an interleukin-1β pathway in systemic lupus erythematosus. Arterioscler. Thromb. Vasc. Biol. 37, 707–716 (2017).
Duffau, P. et al. Platelet CD154 potentiates interferon-α secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci. Transl Med. 2, 47ra63 (2010).
Goules, A. et al. Elevated levels of soluble CD40 ligand (sCD40L) in serum of patients with systemic autoimmune diseases. J. Autoimmun. 26, 165–171 (2006).
Ekdahl, K. N., Ronnblom, L., Sturfelt, G. & Nilsson, B. Increased phosphate content in complement component C3, fibrinogen, vitronectin, and other plasma proteins in systemic lupus erythematosus: covariation with platelet activation and possible association with thrombosis. Arthritis Rheum. 40, 2178–2186 (1997).
Lang, T., Foote, A., Lee, J. P., Morand, E. F. & Harris, J. MIF: implications in the pathoetiology of systemic lupus erythematosus. Front. Immunol. 6, 577 (2015).
Foote, A. et al. Macrophage migration inhibitory factor in systemic lupus erythematosus. J. Rheumatol. 31, 268–273 (2004).
Devarapu, S. K. et al. Dual blockade of the pro-inflammatory chemokine CCL2 and the homeostatic chemokine CXCL12 is as effective as high dose cyclophosphamide in murine proliferative lupus nephritis. Clin. Immunol. 169, 139–147 (2016).
Hrycek, E., Franek, A., Blaszczak, E., Dworak, J. & Hrycek, A. Serum levels of selected chemokines in systemic lupus erythematosus patients. Rheumatol. Int. 33, 2423–2427 (2013).
Wang, A. et al. Dysregulated expression of CXCR4/CXCL12 in subsets of patients with systemic lupus erythematosus. Arthritis Rheum. 62, 3436–3446 (2010).
Li, Y. et al. Urinary biomarkers in lupus nephritis. Autoimmun. Rev. 5, 383–388 (2006).
Patsouras, M. D. et al. Elevated expression of platelet-derived chemokines in patients with antiphospholipid syndrome. J. Autoimmun. 65, 30–37 (2015).
Boilard, E. Platelet-derived interleukin-1β fuels the fire in blood vessels in systemic lupus erythematosus. Arterioscler. Thromb. Vasc. Biol. 37, 607–608 (2017).
Lood, C. et al. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood 116, 1951–1957 (2010).
Aslan, J. E. et al. Histone deacetylase 6-mediated deacetylation of α-tubulin coordinates cytoskeletal and signaling events during platelet activation. Am. J. Physiol. Cell Physiol. 305, C1230–C1239 (2013).
Cerecedo, D., Martinez-Vieyra, I., Alonso-Rangel, L., Benitez-Cardoza, C. & Ortega, A. Epithelial sodium channel modulates platelet collagen activation. Eur. J. Cell Biol. 93, 127–136 (2014).
Pretorius, E., du Plooy, J., Soma, P. & Gasparyan, A. Y. An ultrastructural analysis of platelets, erythrocytes, white blood cells, and fibrin network in systemic lupus erythematosus. Rheumatol Int. 34, 1005–1009 (2014).
Lood, C. et al. Decreased platelet size is associated with platelet activation and anti-phospholipid syndrome in systemic lupus erythematosus. Rheumatology (Oxford) 56, 408–416 (2017).
Delgado-Garcia, G. et al. Mean platelet volume is decreased in adults with active lupus disease. Revista Brasileira Reumatol. 56, 504–508 (2016).
Bai, M. et al. Mean platelet volume could reflect disease activity of adult patients with systemic lupus erythematosus. Clin. Lab. 62, 1317–1322 (2016).
Leader, A., Pereg, D. & Lishner, M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann. Med. 44, 805–816 (2012).
Leytin, V. Apoptosis in the anucleate platelet. Blood Rev. 26, 51–63 (2012).
White, S. & Rosen, A. Apoptosis in systemic lupus erythematosus. Curr. Opin. Rheumatol 15, 557–562 (2003).
Sellam, J. et al. Increased levels of circulating microparticles in primary Sjögren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res. Ther. 11, R156 (2009).
Pereira, J. et al. Circulating platelet-derived microparticles in systemic lupus erythematosus. Association with increased thrombin generation and procoagulant state. Thromb. Haemost. 95, 94–99 (2006).
Jagroop, I. A. & Mikhailidis, D. P. Angiotensin II can induce and potentiate shape change in human platelets: effect of losartan. J. Hum. Hypertension 14, 581–585 (2000).
Kucera, M. et al. The effects of atorvastatin treatment on the mean platelet volume and red cell distribution width in patients with dyslipoproteinemia and comparison with plasma atherogenicity indicators — a pilot study. Clin. Biochem. 48, 557–561 (2015).
Sivri, N. et al. Statins decrease mean platelet volume irrespective of cholesterol lowering effect. Kardiol. Polska 71, 1042–1047 (2013).
Varol, E. & Ozaydin, M. Mean platelet volume as a surrogate marker of inflammation in systemic lupus erythematosus. Clin. Rheumatol 33, 1691–1692 (2014).
Tan, E. M. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25, 1271–1277 (1982).
Petri, M. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012).
Abdel Galil, S. M. et al. Prognostic significance of platelet count in SLE patients. Platelets 28, 203–207 (2017).
Cervera, R. et al. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European Working Party on Systemic Lupus Erythematosus. Medicine 78, 167–175 (1999).
Keeling, D. M. & Isenberg, D. A. Haematological manifestations of systemic lupus erythematosus. Blood Rev. 7, 199–207 (1993).
Fayyaz, A. et al. Haematological manifestations of lupus. Lupus Sci. Med. 2, e000078 (2015).
Reveille, J. D., Bartolucci, A. & Alarcon, G. S. Prognosis in systemic lupus erythematosus. Negative impact of increasing age at onset, black race, and thrombocytopenia, as well as causes of death. Arthritis Rheum. 33, 37–48 (1990).
Zhao, H., Li, S. & Yang, R. Thrombocytopenia in patients with systemic lupus erythematosus: significant in the clinical implication and prognosis. Platelets 21, 380–385 (2010).
Fernandez, M. et al. Systemic lupus erythematosus in a multiethnic US cohort: XLIII. The significance of thrombocytopenia as a prognostic factor. Arthritis Rheum. 56, 614–621 (2007).
Feinglass, E. J., Arnett, F. C., Dorsch, C. A., Zizic, T. M. & Stevens, M. B. Neuropsychiatric manifestations of systemic lupus erythematosus: diagnosis, clinical spectrum, and relationship to other features of the disease. Medicine 55, 323–339 (1976).
Miller, M. H., Urowitz, M. B. & Gladman, D. D. The significance of thrombocytopenia in systemic lupus erythematosus. Arthritis Rheum. 26, 1181–1186 (1983).
Pujol, M., Ribera, A., Vilardell, M., Ordi, J. & Feliu, E. High prevalence of platelet autoantibodies in patients with systemic lupus erythematosus. Br. J. Haematol. 89, 137–141 (1995).
Fabris, F., Casonato, A., Randi, M. L., Luzzatto, G. & Girolami, A. Clinical significance of surface and internal pools of platelet-associated immunoglobulins in immune thrombocytopenia. Scand. J. Haematol. 37, 215–220 (1986).
Howe, S. E. & Lynch, D. M. Platelet antibody binding in systemic lupus erythematosus. J. Rheumatol 14, 482–486 (1987).
Hisada, R. et al. Thrombotic risk stratification by platelet count in patients with antiphospholipid antibodies: a longitudinal study. J. Thromb. Haemost. 15, 1782–1787 (2017).
Michel, M. et al. The spectrum of Evans syndrome in adults: new insight into the disease based on the analysis of 68 cases. Blood 114, 3167–3172 (2009).
Zufferey, A., Kapur, R. & Semple, J. W. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J. Clin. Med. 6, 16 (2017).
Fabris, F. et al. Specific antiplatelet autoantibodies in patients with antiphospholipid antibodies and thrombo-cytopenia. Eur. J. Haematol. 53, 232–236 (1994).
Qian, K. et al. Functional expression of IgA receptor FcαRI on human platelets. J. Leukoc. Biol. 84, 1492–1500 (2008).
Qiao, J., Al-Tamimi, M., Baker, R. I., Andrews, R. K. & Gardiner, E. E. The platelet Fc receptor, FcγRIIa. Immunol. Rev. 268, 241–252 (2015).
Reveille, J. D. The genetic basis of autoantibody production. Autoimmun Rev. 5, 389–398 (2006).
Balada, E. et al. Multiplex family-based study in systemic lupus erythematosus: association between the R620W polymorphism of PTPN22 and the FcγRIIa (CD32A) R131 allele. Tissue Antigens 68, 432–438 (2006).
Arman, M. & Krauel, K. Human platelet IgG Fc receptor FcγRIIA in immunity and thrombosis. J. Thromb. Haemost. 13, 893–908 (2015).
Huang, Z. Y., Chien, P., Indik, Z. K. & Schreiber, A. D. Human platelet FcγRIIA and phagocytes in immune-complex clearance. Mol. Immunol. 48, 691–696 (2011).
Worth, R. G. et al. Platelet FcγRIIA binds and internalizes IgG-containing complexes. Exp. Hematol. 34, 1490–1495 (2006).
Tsokos, G. C., Lo, M. S., Reis, P. C. & Sullivan, K. E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730 (2016).
Mayadas, T. N., Tsokos, G. C. & Tsuboi, N. Mechanisms of immune complex-mediated neutrophil recruitment and tissue injury. Circulation 120, 2012–2024 (2009).
Reilly, M. P. et al. Heparin-induced thrombocytopenia/thrombosis in a transgenic mouse model requires human platelet factor 4 and platelet activation through FcγRIIA. Blood 98, 2442–2447 (2001).
Boilard, E. et al. Influenza virus H1N1 activates platelets through FcγRIIA signaling and thrombin generation. Blood 123, 2854–2863 (2014).
Arman, M. et al. Amplification of bacteria-induced platelet activation is triggered by FcγRIIA, integrin αIIbβ3, and platelet factor 4. Blood 123, 3166–3174 (2014).
Berlacher, M. D. et al. FcγRIIa ligation induces platelet hypersensitivity to thrombotic stimuli. Am. J. Pathol. 182, 244–254 (2013).
Johnson, R. J. et al. Platelets mediate neutrophil-dependent immune complex nephritis in the rat. J. Clin. Invest. 82, 1225–1235 (1988).
Zoja, C. & Remuzzi, G. Role of platelets in progressive glomerular diseases. Pediatr. Nephrol. 9, 495–502 (1995).
Hamad, O. A. et al. Complement component C3 binds to activated normal platelets without preceding proteolytic activation and promotes binding to complement receptor 1. J. Immunol. 184, 2686–2692 (2010).
Saggu, G. et al. Identification of a novel mode of complement activation on stimulated platelets mediated by properdin and C3(H2O). J. Immunol. 190, 6457–6467 (2013).
Peerschke, E. I., Yin, W., Grigg, S. E. & Ghebrehiwet, B. Blood platelets activate the classical pathway of human complement. J. Thromb. Haemost. 4, 2035–2042 (2006).
Peerschke, E. I., Murphy, T. K. & Ghebrehiwet, B. Activation-dependent surface expression of gC1qR/p33 on human blood platelets. Thromb. Haemost. 89, 331–339 (2003).
Peerschke, E. I., Yin, W. & Ghebrehiwet, B. Complement activation on platelets: implications for vascular inflammation and thrombosis. Mol. Immunol. 47, 2170–2175 (2010).
Del Conde, I., Cruz, M. A., Zhang, H., Lopez, J. A. & Afshar-Kharghan, V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 201, 871–879 (2005).
Schmaier, A. H., Amenta, S., Xiong, T., Heda, G. D. & Gewirtz, A. M. Expression of platelet C1 inhibitor. Blood 82, 465–474 (1993).
Hamad, O. A. et al. Contribution of chondroitin sulfate A to the binding of complement proteins to activated platelets. PLoS ONE 5, e12889 (2010).
Peerschke, E. I. & Ghebrehiwet, B. C1q augments platelet activation in response to aggregated Ig. J. Immunol. 159, 5594–5598 (1997).
Lood, C. et al. Increased C1q, C4 and C3 deposition on platelets in patients with systemic lupus erythematosus — a possible link to venous thrombosis? Lupus 21, 1423–1432 (2012).
Murata, K. & Baldwin, W. M. 3rd. Mechanisms of complement activation, C4d deposition, and their contribution to the pathogenesis of antibody-mediated rejection. Transplant. Rev. 23, 139–150 (2009).
Navratil, J. S. et al. Platelet C4d is highly specific for systemic lupus erythematosus. Arthritis Rheum. 54, 670–674 (2006).
Peerschke, E. I. et al. Serum complement activation on heterologous platelets is associated with arterial thrombosis in patients with systemic lupus erythematosus and antiphospholipid antibodies. Lupus 18, 530–538 (2009).
Ziakas, P. D., Poulou, L. S., Giannouli, S., Tzioufas, A. G. & Voulgarelis, M. Thrombocytopenia in lupus: baseline C3 as an independent risk factor for relapse. Ann. Rheum. Dis. 66, 130–131 (2007).
Baroni, G. et al. The role of platelets in antiphospholipid syndrome. Platelets 28, 762–766 (2017).
Urbanus, R. T., Pennings, M. T., Derksen, R. H. & de Groot, P. G. Platelet activation by dimeric β2-glycoprotein I requires signaling via both glycoprotein Ibα and apolipoprotein E receptor 2'. J. Thromb. Haemost. 6, 1405–1412 (2008).
Shi, T. et al. Anti-β2-glycoprotein I antibodies in complex with β2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum. 54, 2558–2567 (2006).
Khamashta, M. A. et al. Immune mediated mechanism for thrombosis: antiphospholipid antibody binding to platelet membranes. Ann. Rheum. Dis. 47, 849–854 (1988).
Lood, C. et al. Platelet activation and anti-phospholipid antibodies collaborate in the activation of the complement system on platelets in systemic lupus erythematosus. PLoS ONE 9, e99386 (2014).
Pierangeli, S. S., Vega-Ostertag, M., Liu, X. & Girardi, G. Complement activation: a novel pathogenic mechanism in the antiphospholipid syndrome. Ann. NY Acad. Sci. 1051, 413–420 (2005).
Jonsson, G. et al. Rheumatological manifestations, organ damage and autoimmunity in hereditary C2 deficiency. Rheumatology (Oxford) 46, 1133–1139 (2007).
Rodriguez-Pinto, I., Espinosa, G. & Cervera, R. Catastrophic antiphospholipid syndrome: the current management approach. Best Pract. Res. Clin. Rheumatol. 30, 239–249 (2016).
Lonze, B. E. et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am. J. Transplant 14, 459–465 (2014).
Levine, J. S. et al. Phospholipid-binding proteins differ in their capacity to induce autoantibodies and murine systemic lupus erythematosus. Lupus 23, 752–768 (2014).
Chighizola, C. B., Gerosa, M. & Meroni, P. L. New tests to detect antiphospholipid antibodies: anti-domain I β-2-glycoprotein-I antibodies. Curr. Rheumatol. Rep. 16, 402 (2014).
Meroni, P. L. Anti-β-2 glycoprotein I epitope specificity: from experimental models to diagnostic tools. Lupus 25, 905–910 (2016).
Kawai, T. & Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637–650 (2011).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Boudreau, L. H. et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood 124, 2173–2183 (2014).
Vogel, S. et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J. Clin. Invest. 125, 4638–4654 (2015).
Wang, Y. et al. Platelet-derived S100 family member myeloid-related protein-14 regulates thrombosis. J. Clin. Invest. 124, 2160–2171 (2014).
Deane, J. A. et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810 (2007).
D'Atri, L. P. et al. Expression and functionality of Toll-like receptor 3 in the megakaryocytic lineage. J. Thromb. Haemost. 13, 839–850 (2015).
Cognasse, F. et al. The inflammatory role of platelets via their TLRs and Siglec receptors. Front. Immunol. 6, 83 (2015).
Koupenova, M. et al. Platelet-TLR7 mediates host survival and platelet count during viral infection in the absence of platelet-dependent thrombosis. Blood 124, 791–802 (2014).
Koupenova, M. et al. Sex differences in platelet Toll-like receptors and their association with cardiovascular risk factors. Arterioscler. Thromb. Vasc. Biol. 35, 1030–1037 (2015).
Yuan, Y. et al. Excessive activation of the TLR9/TGF-β1/PDGF-B pathway in the peripheral blood of patients with systemic lupus erythematosus. Arthritis Res. Ther. 19, 70 (2017).
Berthet, J. et al. Human platelets can discriminate between various bacterial LPS isoforms via TLR4 signaling and differential cytokine secretion. Clin. Immunol. 145, 189–200 (2012).
Jenne, C. N., Urrutia, R. & Kubes, P. Platelets: bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 35, 254–261 (2013).
Villanueva, E. et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 (2011).
Barnado, A., Crofford, L. J. & Oates, J. C. At the bedside: Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J. Leukoc. Biol. 99, 265–278 (2016).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Maugeri, N. et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 12, 2074–2088 (2014).
Ma, C. Y. et al. Elevated plasma level of HMGB1 is associated with disease activity and combined alterations with IFN-α and TNF-α in systemic lupus erythematosus. Rheumatol. Int. 32, 395–402 (2012).
Choi, W. S., Jeon, O. H. & Kim, D. S. CD40 ligand shedding is regulated by interaction between matrix metalloproteinase-2 and platelet integrin αIIbβ3. J. Thromb. Haemost. 8, 1364–1371 (2010).
Rahman, M. et al. Metalloproteinases regulate CD40L shedding from platelets and pulmonary recruitment of neutrophils in abdominal sepsis. Inflamm. Res. 61, 571–579 (2012).
Rahman, M. et al. Platelet shedding of CD40L is regulated by matrix metalloproteinase-9 in abdominal sepsis. J. Thromb. Haemost. 11, 1385–1398 (2013).
Elgueta, R. et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229, 152–172 (2009).
Phipps, R. P., Kaufman, J. & Blumberg, N. Platelet derived CD154 (CD40 ligand) and febrile responses to transfusion. Lancet 357, 2023–2024 (2001).
Henn, V. et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391, 591–594 (1998).
Cognasse, F. et al. Human platelets can activate peripheral blood B cells and increase production of immunoglobulins. Exp. Hematol. 35, 1376–1387 (2007).
Elzey, B. D. et al. Platelet-derived CD154 enables T-cell priming and protection against Listeria monocytogenes challenge. Blood 111, 3684–3691 (2008).
Ntelis, K., Solomou, E. E., Sakkas, L., Liossis, S. N. & Daoussis, D. The role of platelets in autoimmunity, vasculopathy, and fibrosis: implications for systemic sclerosis. Semin. Arthritis Rheum. 47, 409–417 (2017).
Elzey, B. D. et al. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J. Leukoc. Biol. 78, 80–84 (2005).
de Sanctis, J. B., Garmendia, J. V., Chaurio, R., Zabaleta, M. & Rivas, L. Total and biologically active CD154 in patients with SLE. Autoimmunity 42, 263–265 (2009).
Cote, F. et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc. Natl Acad. Sci. USA 100, 13525–13530 (2003).
Mercado, C. P. & Kilic, F. Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels. Mol. Interv 10, 231–241 (2010).
Angiolillo, D. J., Ueno, M. & Goto, S. Basic principles of platelet biology and clinical implications. Circul. J. 74, 597–607 (2010).
Herr, N., Bode, C. & Duerschmied, D. The effects of serotonin in immune cells. Front. Cardiovasc. Med. 4, 48 (2017).
Bennett, A., Frampton, G. & Cameron, J. S. Platelet-associated IgG in idiopathic glomerulonephritis and the nephritis of systemic lupus erythematosus. Br. J. Haematol. 62, 695–703 (1986).
Dorsch, C. A. & Meyerhoff, J. Mechanisms of abnormal platelet aggregation in systemic lupus erythematosus. Arthritis Rheum. 25, 966–973 (1982).
Kanai, H., Tsuchida, A., Yano, S. & Naruse, T. Intraplatelet and urinary serotonin concentrations in systemic lupus erythematosus with reference to its clinical manifestations. J. Med. 20, 371–390 (1989).
Lood, C. et al. Type I interferon-mediated skewing of the serotonin synthesis is associated with severe disease in systemic lupus erythematosus. PLoS ONE 10, e0125109 (2015).
Meyerhoff, J. & Dorsch, C. A. Decreased platelet serotonin levels in systemic lupus erythematosus. Arthritis Rheum. 24, 1495–1500 (1981).
Parbtani, A., Frampton, G., Yewdall, V., Kasai, N. & Cameron, J. S. Platelet and plasma serotonin in glomerulonephritis. III: The nephritis of systemic lupus erythematosus. Clin. Nephrol. 14, 164–172 (1980).
Zeller, J., Weissbarth, E., Baruth, B., Mielke, H. & Deicher, H. Serotonin content of platelets in inflammatory rheumatic diseases. Correlation with clinical activity. Arthritis Rheum. 26, 532–540 (1983).
Praschak-Rieder, N. et al. Novel 5-HTTLPR allele associates with higher serotonin transporter binding in putamen: a [11C] DASB positron emission tomography study. Biol. Psychiatry 62, 327–331 (2007).
Denny, M. F. et al. Interferon-α promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood 110, 2907–2915 (2007).
Kaplan, M. J. Premature vascular damage in systemic lupus erythematosus. Autoimmunity 42, 580–586 (2009).
Gustafsson, J. T. et al. Excess atherosclerosis in systemic lupus erythematosus, — a matter of renal involvement: case control study of 281 SLE patients and 281 individually matched population controls. PLoS ONE 12, e0174572 (2017).
Stalker, T. J. et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood 121, 1875–1885 (2013).
Furie, B. & Furie, B. C. Mechanisms of thrombus formation. N. Engl. J. Med. 359, 938–949 (2008).
Vikerfors, A. et al. Clinical manifestations and anti-phospholipid antibodies in 712 patients with systemic lupus erythematosus: evaluation of two diagnostic assays. Rheumatology (Oxford) 52, 501–509 (2013).
Macchi, L. et al. Anti-platelet antibodies in patients with systemic lupus erythematosus and the primary antiphospholipid antibody syndrome: their relationship with the observed thrombocytopenia. Br. J. Haematol. 98, 336–341 (1997).
Weissbarth, E., Baruth, B., Mielke, H., Liman, W. & Deicher, H. Platelets as target cells in rheumatoid arthritis and systemic lupus erythematosus: a platelet specific immunoglobulin inducing the release reaction. Rheumatol. Int. 2, 67–73 (1982).
Kasai, N. et al. Platelet-aggregating immune complexes in idiopathic glomerulonephritis and SLE. Proc. Eur. Dial. Transplant. Assoc. 17, 621–628 (1980).
Cloutier, N. et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1720553115 (2018).
Walther, D. J. et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299, 76 (2003).
Averill, M. M., Kerkhoff, C. & Bornfeldt, K. E. S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler. Thromb. Vasc. Biol. 32, 223–229 (2012).
Edgeworth, J., Gorman, M., Bennett, R., Freemont, P. & Hogg, N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J. Biol. Chem. 266, 7706–7713 (1991).
Yen, T. et al. Induction of the S100 chemotactic protein, CP-10, in murine microvascular endothelial cells by proinflammatory stimuli. Blood 90, 4812–4821 (1997).
Vogl, T. et al. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 13, 1042–1049 (2007).
Kerkhoff, C., Sorg, C., Tandon, N. N. & Nacken, W. Interaction of S100A8/S100A9-arachidonic acid complexes with the scavenger receptor CD36 may facilitate fatty acid uptake by endothelial cells. Biochemistry 40, 241–248 (2001).
Hibino, T. et al. S100A9 is a novel ligand of EMMPRIN that promotes melanoma metastasis. Cancer Res. 73, 172–183 (2013).
Loser, K. et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat. Med. 16, 713–717 (2010).
Donato, R. et al. Functions of S100 proteins. Curr. Mol. Med. 13, 24–57 (2013).
Bjork, P. et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 7, e97 (2009).
Payen, D. et al. Gene profiling in human blood leucocytes during recovery from septic shock. Intensive Care Med. 34, 1371–1376 (2008).
Gao, S., Yang, Y., Fu, Y., Guo, W. & Liu, G. Diagnostic and prognostic value of myeloid-related protein complex 8/14 for sepsis. Am. J. Emerg. Med. 33, 1278–1282 (2015).
Foell, D. & Roth, J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 50, 3762–3771 (2004).
van Lent, P. L. et al. Myeloid-related proteins S100A8/S100A9 regulate joint inflammation and cartilage destruction during antigen-induced arthritis. Ann. Rheum. Dis. 67, 1750–1758 (2008).
Tyden, H. et al. Pro-inflammatory S100 proteins are associated with glomerulonephritis and anti-dsDNA antibodies in systemic lupus erythematosus. Lupus 26, 139–149 (2016).
Tyden, H. et al. Increased serum levels of S100A8/A9 and S100A12 are associated with cardiovascular disease in patients with inactive systemic lupus erythematosus. Rheumatology (Oxford) 52, 2048–2055 (2013).
Soyfoo, M. S., Roth, J., Vogl, T., Pochet, R. & Decaux, G. Phagocyte-specific S100A8/A9 protein levels during disease exacerbations and infections in systemic lupus erythematosus. J. Rheumatol. 36, 2190–2194 (2009).
McCormick, M. M. et al. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J. Biol. Chem. 280, 41521–41529 (2005).
Oesterle, A. & Bowman, M. A. S100A12 and the S100/calgranulins: emerging biomarkers for atherosclerosis and possibly therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 35, 2496–2507 (2015).
Healy, A. M. et al. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation 113, 2278–2284 (2006).
Lood, C. et al. Platelet-derived S100A8/A9 and cardiovascular disease in systemic lupus erythematosus. Arthritis Rheumatol. 68, 1970–1980 (2016).
Nojima, J. et al. Strong correlation between the prevalence of cerebral infarction and the presence of anti-cardiolipin/β2-glycoprotein I and anti-phosphatidylserine/prothrombin antibodies — co-existence of these antibodies enhances ADP-induced platelet activation in vitro. Thromb. Haemost. 91, 967–976 (2004).
Lin, Y. L. & Wang, C. T. Activation of human platelets by the rabbit anticardiolipin antibodies. Blood 80, 3135–3143 (1992).
Vega-Ostertag, M., Harris, E. N. & Pierangeli, S. S. Intracellular events in platelet activation induced by antiphospholipid antibodies in the presence of low doses of thrombin. Arthritis Rheum. 50, 2911–2919 (2004).
Melki, I., Tessandier, N., Zufferey, A. & Boilard, E. Platelet microvesicles in health and disease. Platelets 28, 214–221 (2017).
Heijnen, H. F., Schiel, A. E., Fijnheer, R., Geuze, H. J. & Sixma, J. J. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and α-granules. Blood 94, 3791–3799 (1999).
Tersteeg, C. et al. FLow-induced PRotrusions (FLIPRs): a platelet-derived platform for the retrieval of microparticles by monocytes and neutrophils. Circ. Res. 114, 780–791 (2014).
Arraud, N. et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J. Thromb. Haemost. 12, 614–627 (2014).
Flaumenhaft, R. et al. Megakaryocyte-derived microparticles: direct visualization and distinction from platelet-derived microparticles. Blood 113, 1112–1121 (2009).
Gitz, E. et al. CLEC-2 expression is maintained on activated platelets and on platelet microparticles. Blood 124, 2262–2270 (2014).
Cunin, P. et al. Megakaryocytes compensate for Kit insufficiency in murine arthritis. J. Clin. Invest. 127, 1714–1724 (2017).
Aloui, C. et al. The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int. J. Mol. Sci. 15, 22342–22364 (2014).
Boilard, E., Duchez, A. C. & Brisson, A. The diversity of platelet microparticles. Curr. Opin. Hematol. 22, 437–444 (2015).
Pisetsky, D. S. Microparticles as autoantigens: making immune complexes big. Arthritis Rheum. 64, 958–961 (2012).
Lopez, P., Rodriguez-Carrio, J., Martinez-Zapico, A., Caminal-Montero, L. & Suarez, A. Circulating microparticle subpopulations in systemic lupus erythematosus are affected by disease activity. Int. J. Cardiol. 236, 138–144 (2017).
McCarthy, E. M. et al. Microparticle subpopulations are potential markers of disease progression and vascular dysfunction across a spectrum of connective tissue disease. BBA Clin. 7, 16–22 (2017).
Nielsen, C. T., Ostergaard, O., Johnsen, C., Jacobsen, S. & Heegaard, N. H. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum. 63, 3067–3077 (2011).
Fortin, P. R. et al. Distinct subtypes of microparticle-containing immune complexes are associated with disease activity, damage, and carotid intima-media thickness in systemic lupus erythematosus. J. Rheumatol. 43, 2019–2025 (2016).
Caielli, S. et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 213, 697–713 (2016).
Sandhir, R., Halder, A. & Sunkaria, A. Mitochondria as a centrally positioned hub in the innate immune response. Biochim. Biophys. Acta 1863, 1090–1097 (2017).
Berg, P. A. & Klein, R. Mitochondrial antigens and autoantibodies: from anti-M1 to anti-M9. Klinische Wochenschrift 64, 897–909 (1986).
Wang, H., Li, T., Chen, S., Gu, Y. & Ye, S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 67, 3190–3200 (2015).
Reimer, G., Rubin, R. L., Kotzin, B. L. & Tan, E. M. Anti-native DNA antibodies from autoimmune sera also bind to DNA in mitochondria. J. Immunol. 133, 2532–2536 (1984).
Thachil, J. Platelets in inflammatory disorders: a pathophysiological and clinical perspective. Semin. Thromb. Hemost. 41, 572–581 (2015).
Gawaz, M. et al. Platelets induce alterations of chemotactic and adhesive properties of endothelial cells mediated through an interleukin-1-dependent mechanism. Implications for atherogenesis. Atherosclerosis 148, 75–85 (2000).
Kaplanski, G. et al. Activated platelets induce endothelial secretion of interleukin-8 in vitro via an interleukin-1-mediated event. Blood 81, 2492–2495 (1993).
Furie, B. & Furie, B. C. The molecular basis of platelet and endothelial cell interaction with neutrophils and monocytes: role of P-selectin and the P-selectin ligand, PSGL-1. Thromb. Haemost. 74, 224–227 (1995).
Ehlers, R. et al. Targeting platelet-leukocyte interactions: identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibα. J. Exp. Med. 198, 1077–1088 (2003).
Wang, Y. et al. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbα. Nat. Commun. 8, 15559 (2017).
Flick, M. J. et al. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin αMβ2 binding motif. J. Clin. Invest. 117, 3224–3235 (2007).
Flick, M. J. et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor αMβ2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Invest. 113, 1596–1606 (2004).
Flick, M. J. et al. The development of inflammatory joint disease is attenuated in mice expressing the anticoagulant prothrombin mutant W215A/E217A. Blood 117, 6326–6337 (2011).
Joseph, J. E., Harrison, P., Mackie, I. J., Isenberg, D. A. & Machin, S. J. Increased circulating platelet-leucocyte complexes and platelet activation in patients with antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Br. J. Haematol. 115, 451–459 (2001).
Hirahashi, J. et al. Mac-1 (CD11b/CD18) links inflammation and thrombosis after glomerular injury. Circulation 120, 1255–1265 (2009).
Mejia-Vilet, J. M., Cordova-Sanchez, B. M., Uribe-Uribe, N. O., Correa-Rotter, R. & Morales-Buenrostro, L. E. Prognostic significance of renal vascular pathology in lupus nephritis. Lupus 26, 1042–1050 (2017).
Tektonidou, M. G., Sotsiou, F., Nakopoulou, L., Vlachoyiannopoulos, P. G. & Moutsopoulos, H. M. Antiphospholipid syndrome nephropathy in patients with systemic lupus erythematosus and antiphospholipid antibodies: prevalence, clinical associations, and long-term outcome. Arthritis Rheum. 50, 2569–2579 (2004).
Daugas, E. et al. Antiphospholipid syndrome nephropathy in systemic lupus erythematosus. J. Am. Soc. Nephrol. 13, 42–52 (2002).
Petri, B. et al. von Willebrand factor promotes leukocyte extravasation. Blood 116, 4712–4719 (2010).
van Spriel, A. B. et al. Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood 97, 2478–2486 (2001).
Galindo, M. et al. Immunohistochemical detection of intravascular platelet microthrombi in patients with lupus nephritis and anti-phospholipid antibodies. Rheumatology (Oxford) 48, 1003–1007 (2009).
ElGendi, S. S. & El-Sherif, W. T. Anti-C1q antibodies, sCD40L, TWEAK and CD4/CD8 ratio in systemic lupus erythematosus and their relations to disease activity and renal involvement. Egypt. J. Immunol. 16, 135–148 (2009).
Yellin, M. J. et al. Immunohistologic analysis of renal CD40 and CD40L expression in lupus nephritis and other glomerulonephritides. Arthritis Rheum. 40, 124–134 (1997).
Delmas, Y. et al. Activation of mesangial cells by platelets in systemic lupus erythematosus via a CD154-dependent induction of CD40. Kidney Int. 68, 2068–2078 (2005).
de Jong, E. C. et al. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J. Immunol. 168, 1704–1709 (2002).
Wu, T. et al. Elevated urinary VCAM-1, P-selectin, soluble TNF receptor-1, and CXC chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J. Immunol. 179, 7166–7175 (2007).
Ruiz-Irastorza, G. et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 15, 577–583 (2006).
Ho, K. T. et al. Systemic lupus erythematosus in a multiethnic cohort (LUMINA): XXVIII. Factors predictive of thrombotic events. Rheumatology (Oxford) 44, 1303–1307 (2005).
Siso, A. et al. Previous antimalarial therapy in patients diagnosed with lupus nephritis: influence on outcomes and survival. Lupus 17, 281–288 (2008).
Espinola, R. G., Pierangeli, S. S., Gharavi, A. E. & Harris, E. N. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb. Haemost. 87, 518–522 (2002).
Johansson, E., Forsberg, K. & Johnsson, H. Clinical and experimental evaluation of the thromboprophylactic effect of hydroxychloroquine sulfate after total hip replacement. Haemostasis 10, 89–96 (1981).
Cooke, E. D. et al. Failure of orally administered hydroxychloroquine sulphate to prevent venous thromboembolism following elective hip operations. J. Bone Joint Surg. Am. 59, 496–500 (1977).
Fox, R. Anti-malarial drugs: possible mechanisms of action in autoimmune disease and prospects for drug development. Lupus 5 (Suppl. 1), S4–S10 (1996).
Kyburz, D., Brentano, F. & Gay, S. Mode of action of hydroxychloroquine in RA — evidence of an inhibitory effect on Toll-like receptor signaling. Nat. Clin. Pract. Rheumatol. 2, 458–459 (2006).
Bertolaccini, M. L. et al. Complement inhibition by hydroxychloroquine prevents placental and fetal brain abnormalities in antiphospholipid syndrome. J. Autoimmun. 75, 30–38 (2016).
Goldman, F. D. et al. Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties. Blood 95, 3460–3466 (2000).
Lopez, P., Rodriguez-Carrio, J. & Suarez, A. Antimalarial drugs inhibit IFNα-enhanced TNFα and STAT4 expression in monocytes: implication for systemic lupus erythematosus. Cytokine 67, 13–20 (2014).
Prowse, C., Pepper, D. & Dawes, J. Prevention of the platelet α-granule release reaction by membrane-active drugs. Thromb. Res. 25, 219–227 (1982).
McCrea, J. M., Robinson, P. & Gerrard, J. M. Mepacrine (quinacrine) inhibition of thrombin-induced platelet responses can be overcome by lysophosphatidic acid. Biochim. Biophys. Acta 842, 189–194 (1985).
Peters, A. L., Stunz, L. L. & Bishop, G. A. CD40 and autoimmunity: the dark side of a great activator. Semin. Immunol. 21, 293–300 (2009).
Mohan, C., Shi, Y., Laman, J. D. & Datta, S. K. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J. Immunol. 154, 1470–1480 (1995).
Early, G. S., Zhao, W. & Burns, C. M. Anti-CD40 ligand antibody treatment prevents the development of lupus-like nephritis in a subset of New Zealand black x New Zealand white mice. Response correlates with the absence of an anti-antibody response. J. Immunol. 157, 3159–3164 (1996).
Wang, X. et al. Effects of anti-CD154 treatment on B cells in murine systemic lupus erythematosus. Arthritis Rheum. 48, 495–506 (2003).
Quezada, S. A. et al. Distinct mechanisms of action of anti-CD154 in early versus late treatment of murine lupus nephritis. Arthritis Rheum. 48, 2541–2554 (2003).
Boumpas, D. T. et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 48, 719–727 (2003).
Huang, W. et al. The effect of anti-CD40 ligand antibody on B cells in human systemic lupus erythematosus. Arthritis Rheum. 46, 1554–1562 (2002).
Law, C. L. & Grewal, I. S. Therapeutic interventions targeting CD40L (CD154) and CD40: the opportunities and challenges. Adv. Exp. Med. Biol. 647, 8–36 (2009).
Kalunian, K. C. et al. Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD154: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 46, 3251–3258 (2002).
Chamberlain, C. et al. Repeated administration of dapirolizumab pegol in a randomised phase I study is well tolerated and accompanied by improvements in several composite measures of systemic lupus erythematosus disease activity and changes in whole blood transcriptomic profiles. Ann. Rheum. Dis. 76, 1837–1844 (2017).
Bishop-Bailey, D. The platelet as a model system for the acute actions of nuclear receptors. Steroids 75, 570–575 (2010).
Liverani, E., Banerjee, S., Roberts, W., Naseem, K. M. & Perretti, M. Prednisolone exerts exquisite inhibitory properties on platelet functions. Biochem. Pharmacol. 83, 1364–1373 (2012).
Dinarello, C. A. Anti-inflammatory agents: present and future. Cell 140, 935–950 (2010).
Jain, M. K. & Ridker, P. M. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat. Rev. Drug Discov. 4, 977–987 (2005).
Artola, R. T., Mihos, C. G. & Santana, O. Effects of statin therapy in patients with systemic lupus erythematosus. South. Med. J. 109, 705–711 (2016).
Cipollone, F. et al. Association between enhanced soluble CD40L and prothrombotic state in hypercholesterolemia: effects of statin therapy. Circulation 106, 399–402 (2002).
Semb, A. G. et al. Raised serum levels of soluble CD40 ligand in patients with familial hypercholesterolemia: downregulatory effect of statin therapy. J. Am. Coll. Cardiol. 41, 275–279 (2003).
Sanguigni, V. et al. Short-term treatment with atorvastatin reduces platelet CD40 ligand and thrombin generation in hypercholesterolemic patients. Circulation 111, 412–419 (2005).
Ferroni, P., Basili, S., Santilli, F. & Davi, G. Low-density lipoprotein-lowering medication and platelet function. Pathophysiol Haemost. Thromb. 35, 346–354 (2006).
Watanabe, T. et al. Effects of statins on thrombosis development in patients with systemic lupus erythematosus and antiphospholipid antibodies. Lupus 27, 225–234 (2017).
de Abajo, F. J. Effects of selective serotonin reuptake inhibitors on platelet function: mechanisms, clinical outcomes and implications for use in elderly patients. Drugs Aging 28, 345–367 (2011).
Krishnadas, R. & Cavanagh, J. Sustained remission of rheumatoid arthritis with a specific serotonin reuptake inhibitor antidepressant: a case report and review of the literature. J. Med. Case Rep. 5, 112 (2011).
Cloutier, N. et al. Platelets can enhance vascular permeability. Blood 120, 1334–1343 (2012).
Thomas, M. R. & Storey, R. F. Effect of P2Y12 inhibitors on inflammation and immunity. Thromb. Haemost. 114, 490–497 (2015).
Wang, L. et al. Transcriptional down-regulation of the platelet ADP receptor P2Y(12) and clusterin in patients with systemic lupus erythematosus. J. Thromb. Haemost. 2, 1436–1442 (2004).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02320357 (2017).
Valdes, V., Nardi, M. A., Elbaum, L. & Berger, J. S. Reproducibility over time and effect of low-dose aspirin on soluble P-selectin and soluble CD40 ligand. J. Thromb. Thrombolysis 40, 83–87 (2015).
Iudici, M. et al. Low-dose aspirin as primary prophylaxis for cardiovascular events in systemic lupus erythematosus: a long-term retrospective cohort study. Rheumatology (Oxford) 55, 1623–1630 (2016).
Fasano, S. et al. Primary prevention of cardiovascular disease in patients with systemic lupus erythematosus: case series and literature review. Lupus 26, 1463–1472 (2017).
Lit, L. C., Wong, C. K., Tam, L. S., Li, E. K. & Lam, C. W. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 65, 209–215 (2006).
Robak, E., Kulczycka, L., Sysa-Jedrzejowska, A., Wierzbowska, A. & Robak, T. Circulating proangiogenic molecules PIGF, SDF-1 and sVCAM-1 in patients with systemic lupus erythematosus. Eur. Cytokine Netw. 18, 181–187 (2007).
Vakkalanka, R. K. et al. Elevated levels and functional capacity of soluble CD40 ligand in systemic lupus erythematosus sera. Arthritis Rheum. 42, 871–881 (1999).
Lee, Y. H. & Bae, S. C. Association between circulating transforming growth factor-β1 level and polymorphisms in systemic lupus erythematosus and rheumatoid arthritis: a meta-analysis. Cell. Mol. Biol. 63, 53–59 (2017).
Becker-Merok, A., Eilertsen, G. O. & Nossent, J. C. Levels of transforming growth factor-β are low in systemic lupus erythematosus patients with active disease. J. Rheumatol. 37, 2039–2045 (2010).
Abdulahad, D. A. et al. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res. Ther. 13, R71 (2011).
Li, J. et al. Expression of high mobility group box chromosomal protein 1 and its modulating effects on downstream cytokines in systemic lupus erythematosus. J. Rheumatol. 37, 766–775 (2010).
Lee, Y. H., Choi, S. J., Ji, J. D. & Song, G. G. Association between Toll-like receptor polymorphisms and systemic lupus erythematosus: a meta-analysis update. Lupus 25, 593–601 (2016).
Enevold, C. et al. Single nucleotide polymorphisms in genes encoding Toll-like receptors 7, 8 and 9 in Danish patients with systemic lupus erythematosus. Mol. Biol. Rep. 41, 5755–5763 (2014).
Karassa, F. B., Trikalinos, T. A. & Ioannidis, J. P. A. Role of the Fcγ receptor IIa polymorphism in susceptibility to systemic lupus erythematosus and lupus nephritis: a meta-analysis. Arthritis Rheum. 46, 1563–1571 (2002).
Patrono, C., Garcia Rodriguez, L. A., Landolfi, R. & Baigent, C. Low-dose aspirin for the prevention of atherothrombosis. N. Engl. J. Med. 353, 2373–2383 (2005).
Author information
Authors and Affiliations
Contributions
E.B. and C.P.L. researched data for the article. E.B., C.P.L. and C.J.L. wrote the article. All authors made substantial contributions to discussions of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Linge, P., Fortin, P., Lood, C. et al. The non-haemostatic role of platelets in systemic lupus erythematosus. Nat Rev Rheumatol 14, 195–213 (2018). https://doi.org/10.1038/nrrheum.2018.38
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2018.38
This article is cited by
-
Anti-Toxoplasma gondii antibodies as a risk factor for the prevalence and severity of systemic lupus erythematosus
Parasites & Vectors (2024)
-
Modeling of clinical phenotypes in systemic lupus erythematosus based on the platelet transcriptome and FCGR2a genotype
Journal of Translational Medicine (2023)
-
The role of platelets in immune-mediated inflammatory diseases
Nature Reviews Immunology (2023)
-
Pathogenic cellular and molecular mediators in lupus nephritis
Nature Reviews Nephrology (2023)
-
Assessment of peripapillary retinal nerve fiber layer thickness and vessel density in newly diagnosed SLE patients without ocular symptoms
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)