Key Points
-
Inflammatory mediators can directly and indirectly induce pain
-
Pain in ankylosing spondylitis (AS) is not strictly inflammatory and involves a neuropathic component
-
Brain abnormalities that underlie chronic pain can be detected in AS
-
An individual's sex affects the degree of neuro-immune involvement in pain, and sex differences in pain perception might contribute to sexual dimorphisms in AS presentation
-
Pain in AS might involve distinct molecular underpinnings and so strategies to treat pain might differ from those that target inflammation
Abstract
Clinicians have commonly differentiated chronic back pain into two broad subsets: namely, non-inflammatory (or mechanical) back pain and inflammatory back pain. As the terminology suggests, the latter category, in which ankylosing spondylitis (AS) is prominent, presupposes a close link between pain and inflammation. Advances in research into the genetics and immunology of AS have improved our understanding of the inflammatory processes involved in this disease, and have led to the development of potent anti-inflammatory biologic therapeutic agents. However, evidence from clinical trials and from biomarker and imaging studies in patients with AS indicate that pain and inflammation are not always correlated. Thus, the assumption that pain in AS is a reliable surrogate marker for inflammation might be an over-simplification. This Review provides an overview of current concepts relating to neuro-immune interactions in AS and summarizes research that reveals an increasingly complex interplay between the activation of the immune system and pain pathways in the nervous system. The different types of pain experienced by patients with AS, insights from brain imaging studies, neurological mechanisms of pain, sex bias in pain and how the immune system can modify pain in patients with AS are also discussed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Costello, M.-E. et al. Brief report: intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol. 67, 686–691 (2015).
Tsui, F. W., Tsui, H. W., Akram, A., Haroon, N. & Inman, R. D. The genetic basis of ankylosing spondylitis: new insights into disease pathogenesis. Appl. Clin. Genet. 7, 105–115 (2014).
Brown, M. A., Kenna, T. & Wordsworth, B. P. Genetics of ankylosing spondylitis — insights into pathogenesis. Nat. Rev. Rheumatol. 12, 81–91 (2015).
Cortes, A. et al. Major histocompatibility complex associations of ankylosing spondylitis are complex and involve further epistasis with ERAP1. Nat. Commun. 6, 7146 (2015).
Ranganathan, V., Gracey, E., Brown, M. A., Inman, R. D. & Haroon, N. Pathogenesis of ankylosing spondylitis — recent advances and future directions. Nat. Rev. Rheumatol. 13, 359–367 (2017).
Jethwa, H. & Bowness, P. The interleukin (IL)-23/IL-17 axis in ankylosing spondylitis: new advances and potentials for treatment. Clin. Exp. Immunol. 183, 30–36 (2016).
Taurog, J. D., Chhabra, A. & Colbert, R. A. Ankylosing spondylitis and axial spondyloarthritis. N. Engl. J. Med. 374, 2563–2574 (2016).
Sieper, J. & Poddubnyy, D. New evidence on the management of spondyloarthritis. Nat. Rev. Rheumatol. 12, 282–295 (2016).
Gracey, E. et al. Sexual dimorphism in the Th17 signature of ankylosing spondylitis. Arthritis Rheumatol. 68, 679–689 (2016).
Blachier, M. et al. Factors associated with radiographic lesions in early axial spondyloarthritis. Results from the DESIR cohort. Rheumatology (Oxford) 52, 1686–1693 (2013).
Sieper, J. et al. Sarilumab for the treatment of ankylosing spondylitis: results of a phase II, randomised, double-blind, placebo-controlled study (ALIGN). Ann. Rheum. Dis. 74, 1051–1057 (2015).
Sieper, J., Porter-Brown, B., Thompson, L., Harari, O. & Dougados, M. Assessment of short-term symptomatic efficacy of tocilizumab in ankylosing spondylitis: results of randomised, placebo-controlled trials. Ann. Rheum. Dis. 73, 95–100 (2014).
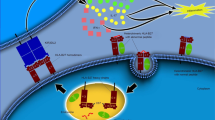
Ji, R.-R., Chamessian, A. & Zhang, Y.-Q. Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577 (2016).
Pinho-Ribeiro, F. A., Verri, W. A. & Chiu, I. M. Nociceptor sensory neuron–immune interactions in pain and inflammation. Trends Immunol. 38, 5–19 (2017).
Wu, Q., Inman, R. D. & Davis, K. D. Neuropathic pain in ankylosing spondylitis: a psychophysics and brain imaging study. Arthritis Rheum. 65, 1494–1503 (2013).
Braun, J. & Inman, R. Clinical significance of inflammatory back pain for diagnosis and screening of patients with axial spondyloarthritis. Ann. Rheum. Dis. 69, 1264–1268 (2010).
Nhan, D. T. & Caplan, L. Patient-reported outcomes in axial spondyloarthritis. Rheum. Dis. Clin. North Am. 42, 285–299 (2016).
Chen, C., Zhang, X., Xiao, L., Zhang, X. & Ma, X. Comparative effectiveness of biologic therapy regimens for ankylosing spondylitis: a systematic review and a network meta-analysis. Medicine (Baltimore) 95, e3060 (2016).
Dagfinrud, H., Vollestad, N. K., Loge, J. H., Kvien, T. K. & Mengshoel, A. M. Fatigue in patients with ankylosing spondylitis: a comparison with the general population and associations with clinical and self-reported measures. Arthritis Rheum. 53, 5–11 (2005).
Brophy, S. et al. Fatigue in ankylosing spondylitis: treatment should focus on pain management. Semin. Arthritis Rheum. 42, 361–367 (2013).
Dernis-Labous, E., Messow, M. & Dougados, M. Assessment of fatigue in the management of patients with ankylosing spondylitis. Rheumatology (Oxford) 42, 1523–1528 (2003).
Wu, Q., Inman, R. D. & Davis, K. D. Tumor necrosis factor inhibitor therapy in ankylosing spondylitis: differential effects on pain and fatigue and brain correlates. Pain 156, 297–304 (2015).
Bedaiwi, M. et al. Fatigue in ankylosing spondylitis and nonradiographic axial spondyloarthritis: analysis from a longitudinal observation cohort. J. Rheumatol. 42, 2354–2360 (2015).
Christie, A., Dagfinrud, H., Mowinckel, P. & Hagen, K. B. Variation in fatigue may be poorly explained by pain: results from a longitudinal, exploratory study. Rheumatol. Int. 36, 279–282 (2016).
Wu, Q., Inman, R. D. & Davis, K. D. Fatigue in ankylosing spondylitis is associated with the brain networks of sensory salience and attention. Arthritis Rheumatol. 66, 295–303 (2014).
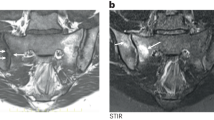
Navarro- Compán, V. et al. Disease activity is longitudinally related to sacroiliac inflammation on MRI in male patients with axial spondyloarthritis: 2-years of the DESIR cohort. Ann. Rheum. Dis. 75, 874–878 (2016).
Baraliakos, X. et al. Quantification of bone marrow edema by magnetic resonance imaging only marginally reflects clinical neck pain evaluation in rheumatoid arthritis and ankylosing spondylitis. J. Rheumatol. 43, 2131–2135 (2016).
Braun, J., Baraliakos, X., Hermann, K.-G. A., Xu, S. & Hsu, B. Serum C-reactive protein levels demonstrate predictive value for radiographic and magnetic resonance imaging outcomes in patients with active ankylosing spondylitis treated with golimumab. J. Rheumatol. 43, 1704–1712 (2016).
Wallis, D., Haroon, N., Ayearst, R., Carty, A. & Inman, R. D. Ankylosing spondylitis and nonradiographic axial spondyloarthritis: part of a common spectrum or distinct diseases? J. Rheumatol. 40, 2038–2041 (2013).
Wach, J., Letroublon, M.-C., Coury, F. & Tebib, J. G. Fibromyalgia in spondyloarthritis: effect on disease activity assessment in clinical practice. J. Rheumatol. 43, 2056–2063 (2016).
Bedaiwi, M. K. et al. Clinical efficacy of celecoxib compared to acetaminophen in chronic nonspecific low back pain: results of a randomized controlled trial. Arthritis Care Res. (Hoboken) 68, 845–852 (2016).
Meesters, J. J. L. et al. The risk for depression in patients with ankylosing spondylitis: a population-based cohort study. Arthritis Res. Ther. 16, 418 (2014).
Van der Horst-Bruinsma, I. E., Zack, D. J., Szumski, A. & Koenig, A. S. Female patients with ankylosing spondylitis: analysis of the impact of gender across treatment studies. Ann. Rheum. Dis. 72, 1221–1224 (2013).
Lee, W. et al. Are there gender differences in severity of ankylosing spondylitis? Results from the PSOAS cohort. Ann. Rheum. Dis. 66, 633–638 (2007).
Karshikoff, B. et al. Modality and sex differences in pain sensitivity during human endotoxemia. Brain. Behav. Immun. 46, 35–43 (2015).
Dodds, K. N. et al. Glial contributions to visceral pain: implications for disease etiology and the female predominance of persistent pain. Transl Psychiatry 6, e888 (2016).
Mogil, J. S. & Chanda, M. L. The case for the inclusion of female subjects in basic science studies of pain. Pain 117, 1–5 (2005).
Rogachov, A. et al. Regional brain signal variability: a novel indicator of pain sensitivity and coping. Pain 157, 2483–2492 (2016).
Wang, G., Erpelding, N. & Davis, K. D. Sex differences in connectivity of the subgenual anterior cingulate cortex. Pain 155, 755–763 (2014).
Hashmi, J. A. & Davis, K. D. Deconstructing sex differences in pain sensitivity. Pain 155, 10–13 (2014).
Hashmi, J. A. & Davis, K. D. Women experience greater heat pain adaptation and habituation than men. Pain 145, 350–357 (2009).
Greenspan, J. D. et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain 132 (Suppl. 1), S26–S45 (2007).
Sorge, R. E. et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 18, 1081–1083 (2015).
Sorge, R. E. et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. 31, 15450–15454 (2011).
Taves, S. et al. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: sex-dependent microglial signaling in the spinal cord. Brain. Behav. Immun. 55, 70–81 (2016).
Draleau, K. et al. Phenotypic identification of spinal cord-infiltrating CD4+ T lymphocytes in a murine model of neuropathic pain. J. Pain Relief Suppl. 3, 3 (2014).
Rosen, S., Ham, B. & Mogil, J. S. Sex differences in neuroimmunity and pain. J. Neurosci. Res. 95, 500–508 (2017).
Varkas, G. & Van den Bosch, F. NSAIDs in axial spondyloarthritis: to be continued...? Ann. Rheum. Dis. 75, 1423–1425 (2016).
Haroon, N., Kim, T.-H. & Inman, R. D. NSAIDs and radiographic progression in ankylosing spondylitis Bagging big game with small arms? Ann. Rheum. Dis. 71, 1593–1595 (2012).
Molto, A., Granger, B., Wendling, D., Dougados, M. & Gossec, L. Use of nonsteroidal anti-inflammatory drugs in early axial spondyloarthritis in daily practice: data from the DESIR cohort. Joint Bone Spine 84, 79–82 (2017).
Varkas, G. et al. Brief report: six-week treatment of axial spondyloarthritis patients with an optimal dose of nonsteroidal antiinflammatory drugs: early response to treatment in signal intensity on magnetic resonance imaging of the sacroiliac joints. Arthritis Rheumatol. 68, 672–678 (2016).
Sieper, J. & Poddubnyy, D. Axial spondyloarthritis. Lancet http://dx.doi.org/10.1016/S0140-6736(16)31591-4 (2017).
Thompson, C., Davies, R. & Choy, E. Anti cytokine therapy in chronic inflammatory arthritis. Cytokine 86, 92–99 (2016).
Hess, A. et al. Blockade of TNF-α rapidly inhibits pain responses in the central nervous system. Proc. Natl Acad. Sci. USA 108, 3731–3736 (2011).
Haroon, N. et al. The impact of TNF-inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 65, 2645–2654 (2013).
Danve, A. & Deodhar, A. Treat to target in axial spondyloarthritis: what are the issues? Curr. Rheumatol. Rep. 19, 22 (2017).
Toussirot, É. Biologics in spondyloarthritis: TNFα inhibitors and other agents. Immunotherapy 7, 669–681 (2015).
Baeten, D. et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N. Engl. J. Med. 373, 2534–2548 (2015).
Sieper, J. New treatment targets for axial spondyloarthritis. Rheumatology (Oxford) 55 (Suppl. 2), ii38–ii42 (2016).
Van der Heijde, D. et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann. Rheum. Dis. 76, 978–991 (2017).
Grace, P. M. et al. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl Acad. Sci. USA 113, E3441–E3450 (2016).
Kistner, K. et al. Systemic desensitization through TRPA1 channels by capsazepine and mustard oil — a novel strategy against inflammation and pain. Sci. Rep. 6, 28621 (2016).
Garrison, S. R. & Stucky, C. L. The dynamic TRPA1 channel: a suitable pharmacological pain target? Curr. Pharm. Biotechnol. 12, 1689–1697 (2011).
Trevisan, G. et al. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 139, 1361–1377 (2016).
Kress, M. et al. MicroRNAs in nociceptive circuits as predictors of future clinical applications. Front. Mol. Neurosci. 6, 33 (2013).
Leinders, M., Üçeyler, N., Pritchard, R. A., Sommer, C. & Sorkin, L. S. Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp. Neurol. 283, 276–286 (2016).
Malfait, A.-M. & Miller, R. J. Emerging targets for the management of osteoarthritis pain. Curr. Osteoporos. Rep. 14, 260–268 (2016).
Siniscalco, D., Giordano, C., Rossi, F., Maione, S. & de Novellis, V. Role of neurotrophins in neuropathic pain. Curr. Neuropharmacol. 9, 523–529 (2011).
Seminowicz, D. A. et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 31, 7540–7550 (2011).
Kucyi, A. & Davis, K. D. The dynamic pain connectome. Trends Neurosci. 38, 86–95 (2015).
Hubbard, C. S. et al. Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro 1, e20.14 (2014).
Mathur, V. A. et al. Altered cognition-related brain activity and interactions with acute pain in migraine. Neuroimage Clin. 7, 347–358 (2015).
Apkarian, A. V. The brain in chronic pain: clinical implications. Pain Manag. 1, 577–586 (2011).
Napadow, V. et al. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 62, 2545–2555 (2010).
Baliki, M. N., Geha, P. Y., Apkarian, A. V. & Chialvo, D. R. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 28, 1398–1403 (2008).
Seminowicz, D. A. & Davis, K. D. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J. Neurophysiol. 97, 3651–3659 (2007).
Davis, K. D. & Moayedi, M. Central mechanisms of pain revealed through functional and structural MRI. J. Neuroimmune Pharmacol. 8, 518–534 (2013).
Goswami, R., Anastakis, D. J., Katz, J. & Davis, K. D. A longitudinal study of pain, personality, and brain plasticity following peripheral nerve injury. Pain 157, 729–739 (2016).
DeSouza, D. D., Davis, K. D. & Hodaie, M. Reversal of insular and microstructural nerve abnormalities following effective surgical treatment for trigeminal neuralgia. Pain 156, 1112–1123 (2015).
Cˇeko, M. et al. Partial recovery of abnormal insula and dorsolateral prefrontal connectivity to cognitive networks in chronic low back pain after treatment. Hum. Brain Mapp. 36, 2075–2092 (2015).
Moayedi, M. et al. Contribution of chronic pain and neuroticism to abnormal forebrain gray matter in patients with temporomandibular disorder. Neuroimage 55, 277–286 (2011).
Moayedi, M. et al. White matter brain and trigeminal nerve abnormalities in temporomandibular disorder. Pain 153, 1467–1477 (2012).
Chen, J. Y.-W., Blankstein, U., Diamant, N. E. & Davis, K. D. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 1392, 121–131 (2011).
Blankstein, U., Chen, J., Diamant, N. E. & Davis, K. D. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology 138, 1783–1789 (2010).
Kucyi, A. et al. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J. Neurosci. 34, 3969–3975 (2014).
Weissman-Fogel, I. et al. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain 152, 384–396 (2011).
Hemington, K. S., Wu, Q., Kucyi, A., Inman, R. D. & Davis, K. D. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct. Funct. 221, 4203–4219 (2016).
Purves, D. et al. (ed.) Neuroscience 2nd edn. (Sinauer Associates, 2001).
Dubin, A. E. & Patapoutian, A. Nociceptors: the sensors of the pain pathway. J. Clin. Invest. 120, 3760–3772 (2010).
Gold, M. S. & Gebhart, G. F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 16, 1248–1257 (2010).
Li, W.-W. et al. Substance P signaling controls mast cell activation, degranulation, and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Anesthesiology 116, 882–895 (2012).
Kim, C. F. & Moalem-Taylor, G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J. Pain 12, 370–383 (2011).
Davis, K. D., Meyer, R. A. & Campbell, J. N. Chemosensitivity and sensitization of nociceptive afferents that innervate the hairy skin of monkey. J. Neurophysiol. 69, 1071–1081 (1993).
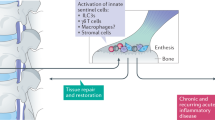
Schaible, H. G. & Grubb, B. D. Afferent and spinal mechanisms of joint pain. Pain 55, 5–54 (1993).
Schaible, H.-G. Nociceptive neurons detect cytokines in arthritis. Arthritis Res. Ther. 16, 470 (2014).
Cervero, F. & Laird, J. M. A. Understanding the signaling and transmission of visceral nociceptive events. J. Neurobiol. 61, 45–54 (2004).
Felson, D. T. The sources of pain in knee osteoarthritis. Curr. Opin. Rheumatol. 17, 624–628 (2005).
Mantyh, P. W. The neurobiology of skeletal pain. Eur. J. Neurosci. 39, 508–519 (2014).
Cavanaugh, J. M., Lu, Y., Chen, C. & Kallakuri, S. Pain generation in lumbar and cervical facet joints. J. Bone Joint Surg. Am. 88 (Suppl. 2), 63–67 (2006).
Szadek, K., Hoogland, P., Zuurmond, W., Delange, J. & Perez, R. Nociceptive nerve fibers in the sacroiliac joint in humans. Reg. Anesth. Pain Med. 33, 36–43 (2008).
Palsson, T. S. & Graven-Nielsen, T. Experimental pelvic pain facilitates pain provocation tests and causes regional hyperalgesia. Pain 153, 2233–2240 (2012).
Rio, E. et al. The pain of tendinopathy: physiological or pathophysiological? Sport. Med. 44, 9–23 (2014).
McCleskey, E. W. & Gold, M. S. Ion channels of nociception. Annu. Rev. Physiol. 61, 835–856 (1999).
Benarroch, E. E. Ion channels in nociceptors: recent developments. Neurology 84, 1153–1164 (2015).
Moore, C. et al. UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc. Natl Acad. Sci. USA 110, E3225–E3234 (2013).
Khodorova, A., Montmayeur, J.-P. & Strichartz, G. Endothelin receptors and pain. J. Pain 10, 4–28 (2009).
Ranade, S. S. et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 (2014).
Cekic, C. & Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 16, 177–192 (2016).
Chiu, I. M., von Hehn, C. A. & Woolf, C. J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 15, 1063–1067 (2012).
Nicotra, L., Loram, L. C., Watkins, L. R. & Hutchinson, M. R. Toll-like receptors in chronic pain. Exp. Neurol. 234, 316–329 (2012).
Meseguer, V. et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 5, 783–801 (2014).
Liu, T., Xu, Z.-Z., Park, C.-K., Berta, T. & Ji, R.-R. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 13, 1460–1462 (2010).
Xu, Z.-Z. et al. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med. 21, 1326–1331 (2015).
Das, N. et al. HMGB1 activates proinflammatory signaling via TLR5 leading to allodynia. Cell Rep. 17, 1128–1140 (2016).
Parsadaniantz, S. M., Rivat, C., Rostène, W. & Goazigo, A. R.-L. Opioid and chemokine receptor crosstalk: a promising target for pain therapy? Nat. Rev. Neurosci. 16, 69–78 (2015).
Zhou, Y.-Q. et al. Interleukin-6: an emerging regulator of pathological pain. J. Neuroinflamm. 13, 141 (2016).
Üçeyler, N., Riediger, N., Kafke, W. & Sommer, C. Differential gene expression of cytokines and neurotrophic factors in nerve and skin of patients with peripheral neuropathies. J. Neurol. 262, 203–212 (2015).
Eskander, M. A. et al. Persistent nociception triggered by nerve growth factor (NGF) is mediated by TRPV1 and oxidative mechanisms. J. Neurosci. 35, 8593–8603 (2015).
Grace, P. M., Hutchinson, M. R., Maier, S. F. & Watkins, L. R. Pathological pain and the neuroimmune interface. Nat. Rev. Immunol. 14, 217–231 (2014).
Vincent, L. et al. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood 122, 1853–1862 (2013).
Vicuña, L. et al. The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell–derived leukocyte elastase. Nat. Med. 21, 518–523 (2015).
Wu, X.-B. et al. CXCL13/CXCR5 enhances sodium channel Nav1.8 current density via p38 MAP kinase in primary sensory neurons following inflammatory pain. Sci. Rep. 6, 34836 (2016).
Latremoliere, A. & Woolf, C. J. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain 10, 895–926 (2009).
Meeus, M. & Nijs, J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin. Rheumatol. 26, 465–473 (2007).
Porreca, F., Ossipov, M. H. & Gebhart, G. F. Chronic pain and medullary descending facilitation. Trends Neurosci. 25, 319–325 (2002).
Dickenson, A. H., Chapman, V. & Green, G. M. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen. Pharmacol. Vasc. Syst. 28, 633–638 (1997).
Tsuda, M. et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424, 778–783 (2003).
Coull, J. A. M. et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 (2005).
Beggs, S., Trang, T. & Salter, M. W. P2X4R+ microglia drive neuropathic pain. Nat. Neurosci. 15, 1068–1073 (2012).
Trang, T., Beggs, S., Wan, X. & Salter, M. W. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J. Neurosci. 29, 3518–3528 (2009).
Coull, J. A. M. et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942 (2003).
Liu, X. J. et al. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat. Med. 14, 1325–1332 (2008).
Salter, M. W. & Kalia, L. V. Src kinases: a hub for NMDA receptor regulation. Nat. Rev. Neurosci. 5, 317–328 (2004).
Keller, A. F., Beggs, S., Salter, M. W. & De Koninck, Y. Transformation of the output of spinal lamina I neurons after nerve injury and microglia stimulation underlying neuropathic pain. Mol. Pain 3, 27 (2007).
Chavan, S. S. & Tracey, K. J. Essential neuroscience in immunology. J. Immunol. 198, 3389–3397 (2017).
Shen, H., Goodall, J. C. & Hill Gaston, J. S. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 60, 1647–1656 (2009).
Smolen, J. S. et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: recommendations of an international task force. Ann. Rheum. Dis. 73, 6–16 (2014).
Task Force on Taxonomy of the International Association for the Study of Pain. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. IASP https://www.iasp-pain.org/files/Content/ContentFolders/Publications2/FreeBooks/Classification-of-Chronic-Pain.pdf (1994).
International Association for the Study of Pain. IASP taxonomy. IASP http://www.iasp-pain.org/Taxonomy (2002).
Williams, A. C. & Craig, K. D. Updating the definition of pain. Pain 157, 2420–2423 (2016).
Bonica, J. (ed.) The Management of Pain (Lea & Febiger, 1953).
Treede, R.-D. et al. A classification of chronic pain for ICD-11. Pain 156, 1 (2015).
Freynhagen, R., Tölle, T. R., Gockel, U. & Baron, R. The painDETECT project — far more than a screening tool on neuropathic pain. Curr. Med. Res. Opin. 32, 1033–1057 (2016).
Hawker, G. A., Mian, S., Kendzerska, T. & French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. (Hoboken) 63, S240–S252 (2011).
Melzack, R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1, 277–299 (1975).
Garrett, S. et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 21, 2286–2291 (1994).
Yarnitsky, D., Granot, M. & Granovsky, Y. Pain modulation profile and pain therapy: between pro- and antinociception. Pain 155, 663–665 (2014).
Tjølsen, A., Lund, A., Berge, O. G. & Hole, K. An improved method for tail-flick testing with adjustment for tail-skin temperature. J. Neurosci. Methods 26, 259–265 (1989).
Hargreaves, K., Dubner, R., Brown, F., Flores, C. & Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32, 77–88 (1988).
Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M. & Yaksh, T. L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 (1994).
Sotocinal, S. G. et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 7, 55 (2011).
Sufka, K. J. Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain 58, 355–366 (1994).
Daou, I. et al. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J. Neurosci. 33, 18631–18640 (2013).
Keystone, E. C., Schorlemmer, H. U., Pope, C. & Allison, A. C. Zymosan-induced arthritis: a model of chronic proliferative arthritis following activation of the alternative pathway of complement. Arthritis Rheum. 20, 1396–1401 (1977).
Courteix, C., Eschalier, A. & Lavarenne, J. Streptozocin-induced diabetic rats: behavioural evidence for a model of chronic pain. Pain 53, 81–88 (1993).
Guan, Z. et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 19, 94–101 (2016).
Hildebrand, M. E. et al. Potentiation of synaptic GluN2B NMDAR currents by Fyn kinase is gated through BDNF-mediated disinhibition in spinal pain processing. Cell Rep. 17, 2753–2765 (2016).
Shubayev, V. I., Strongin, A. Y. & Yaksh, T. L. Role of myelin auto-antigens in pain: a female connection. Neural Regen. Res. 11, 890–891 (2016).
Costigan, M. et al. T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J. Neurosci. 29, 14415–14422 (2009).
Cao, L. & DeLeo, J. A. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur. J. Immunol. 38, 448–458 (2008).
Acknowledgements
The authors would like to acknowledge support in the form of grants from the Canadian Institute of Health Research (CIHR) and the Mayday Fund (to K.D.).
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and undertook review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Dysbiosis
-
A microbial imbalance associated with disease that is typified by a loss of protective bacteria and a gain of pathogenic bacteria.
- Inflammatory back pain
-
A chronic pain of more than 3 months that has a relatively young age of onset (<45 years); morning stiffness is a major symptom associated with inflammation, and unlike mechanical back pain, inflammatory back pain is improved by movement rather than rest.
- Bath Ankylosing Spondylitis Disease Activity Index (BASDAI)
-
A patient-reported outcome that incorporates pain, fatigue and stiffness.
- Sensorimotor network
-
A set of brain regions that includes the primary somatosensory and primary motor cortices; the activity of these regions is related to sensory and/or motor function, and is typically evoked by sensory stimuli or motor tasks.
- Salience network
-
A set of brain regions that function to detect and respond to salient stimuli, such as those that are of value or importance, or simply those that are more prominent than other stimuli.
- Inflammatory pain
-
Pain occurring following an injury or in a disease that involves the release of inflammatory agents that activate and/or sensitize nociceptors.
- Allodynia
-
Pain that is evoked by stimuli that are not normally painful, such as a light touch, gentle heat or mild cooling.
- Central sensitization
-
Enhanced response of the neurons in the central nervous system to normal stimuli.
- Functional brain networks
-
Sets of brain regions for which activity is correlated over time.
- Default mode network
-
A set of brain regions that have ongoing activity during a so-called resting state; this network is thought to monitor ones internal and external environment, and then become suppressed when the individual needs to respond to a situation.
- Functional MRI (fMRI)
-
An MRI-based technique that is used to identify areas of evoked brain responses (for example, responses to a task or stimulus).
- Functional connectivity
-
The degree to which the brain activity in certain regions fluctuates together over time.
- PainDETECT screening tool
-
A screening tool that is used to evaluate the likelihood of the presence of a neuropathic pain component in patients with chronic pain using a questionnaire comprising seven questions that evaluate the quality of pain, sensitivity to light touch or mild temperature, numbness, pain radiation and the temporal pattern of pain.
- Mixed Pain
-
Pain that has multiple components; for example, in ankylosing spondylitis there might be elements of both neuropathic pain and inflammatory pain.
- Peripheral sensitization
-
Increased sensitivity of nociceptors to stimulation resulting from tissue injury.
- Hyperalgesia
-
An increased amount of pain that is evoked by a stimulus that is normally painful.
Rights and permissions
About this article
Cite this article
Bidad, K., Gracey, E., Hemington, K. et al. Pain in ankylosing spondylitis: a neuro-immune collaboration. Nat Rev Rheumatol 13, 410–420 (2017). https://doi.org/10.1038/nrrheum.2017.92
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.92
This article is cited by
-
Neuropathic pain in axial spondyloarthropathy is underdiagnosed and a confounding factor in biologic drug–switching decision: a cross-sectional study
Clinical Rheumatology (2023)
-
A lower frequency of inflammatory back pain in male patients with ankylosing spondylitis compared with female patients
Rheumatology International (2023)
-
Assessment of Neuropathic Pain in Ankylosing Spondylitis: Prevalence and Characteristics
Pain and Therapy (2021)
-
Quality of internet videos related to exercise therapy of ankylosing spondylitis from mainland China
Zeitschrift für Rheumatologie (2021)
-
Interleukin-1β, interleukin-6, and interleukin-17A as indicators reflecting clinical response to celecoxib in ankylosing spondylitis patients
Irish Journal of Medical Science (1971 -) (2021)