Key Points
-
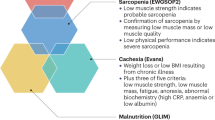
Sarcopenia, the age-related loss of muscle mass and function, is associated with considerable morbidity and health care costs
-
Approaches to defining sarcopenia remain controversial, although several groups have proposed ways of defining the condition
-
Moderate-quality evidence suggests that exercise interventions improve muscle strength and physical performance in patients with sarcopenia, whereas the benefits of nutritional interventions are more equivocal
-
Most pharmacological agents for sarcopenia investigated to date are hormonal (testosterone and selective androgen receptor modulators) although therapies targeting myostatin signalling are emerging as new developments
Abstract
Musculoskeletal ageing is a major public health concern owing to demographic shifts in the population. Sarcopenia, generally defined as the age-related loss of muscle mass and function, is associated with considerable risk of falls, loss of independence in older adults and hospitalization with poorer health outcomes. This condition is therefore associated with increased morbidity and health care costs. As with bone mass, muscle mass and strength increase during late adolescence and early adulthood, but begin to decline substantially from ∼50 years of age. Sarcopenia is characterized by many features, which include loss of muscle mass, altered muscle composition, infiltration with fat and fibrous tissue and alterations in innervation. A better understanding of these factors might help us to develop strategies that target these effects. To date, however, methodological challenges and controversies regarding how best to define the condition, in addition to uncertainty about what outcome measures to consider, have delayed research into possible therapeutic options. Most pharmacological agents investigated to date are hormonal, although new developments have seen the emergence of agents that target myostatin signalling to increase muscle mass. In this review we consider the current approaching for defining sarcopenia and discuss its epidemiology, pathogenesis, and potential therapeutic opportunities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2013 (United Nations, 2013).
Dawson, A. & Dennison, E. Measuring the musculoskeletal aging phenotype. Maturitas 93, 13–17 (2016).
Cooper, C. et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos. Int. 23, 1839–1848 (2012).
Cooper, C. et al. Tools in the assessment of sarcopenia. Calcif. Tissue Int. 93, 201–210 (2013).
Dodds, R. & Aihie Sayer, A. Sarcopenia and frailty: new challenges for clinical practice. Clin. Med. 15 (Suppl. 6), s88–s91 (2015).
McGregor, R., Cameron-Smith, D. & Poppitt, S. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev. Healthspan 3, 9 (2014).
Lang, T. et al. Sarcopenia: etiology, clinical consequences, intervention and assessment. Osteoporos. Int. 21, 543–559 (2010).
Studenski, S. A. et al. The FNIH Sarcopenia project: rationale, study description, conference recommendations and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 69, 547–558 (2014).
Landi, F. et al. Impact of physical function impairment and multimorbidity on mortality among community-living older persons with sarcopenia: results from the ilSIRENTE prospective cohort study. BMJ Open 6, e008281 (2016).
Fried, C. M. et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M156 (2001).
Calvani, R. et al. Biomarkers for physical frailty and sarcopenia: state of the science and future developments. J. Cachexia Sarcopenia Muscle 6, 278–286 (2015).
Dodds, R. M. et al. Grip strength across the life course: normative data from twelve British studies. PLoS ONE 9, e113637 (2014).
Sayer, A. A. et al. The developmental origins of sarcopenia. J. Nutr. Health Aging 12, 427–432 (2008).
Chen, L. K. et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 15, 95–101 (2014).
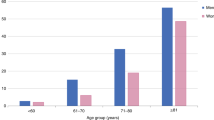
Dodds, R. M. et al. Prevalence and incidence of sarcopenia in the very old: findings from the Newcastle 85+ Study. J. Cachexia Sarcopenia Muscle 8, 229–237 (2016).
Patel, H. P. et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS). Age Ageing 42, 378–384 (2013).
Bortz, W. M. II. A conceptual framework of frailty: a review. J. Gerontol. A Biol. Sci. Med. Sci. 57, M283–M288 (2002).
Animoto, Y. et al. Association of sarcopenia with functional decline in community-dwelling elderly subjects in Japan. Geriatr. Gerontol. Int. 13, 958–963 (2013).
Janssen, I., Baumgartner, R. N., Ross, R., Rosenberg, I. H. & Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 159, 413–421 (2004).
Hardy, S. E., Kang, Y., Studenski, S. A. & Degenholtz, H. B. Ability to walk ¼ mile predicts subsequent disability, mortality and healthcare costs. J. Gen. Intern. Med. 26, 130–135 (2011).
Tanimoto, Y. et al. Sarcopenia and falls in community-dwelling elderly subjects in Japan: defining sarcopenia according to criteria of the European Working Group on Sarcopenia in Older People. Arch. Gerontol. Geriatr. 59, 295–299 (2014).
Sheetz, K. H. et al. Cost of major surgery in the sarcopenic patients. J. Am. Coll. Surg. 217, 813–818 (2013).
Englesbe, M. J. et al. Sarcopenia and mortality after liver transplantation. J. Am. Coll. Surg. 211, 271–278 (2010).
Tan, B. H., Birdsell, L. A., Martin, L., Baracos, V. E. & Fearon, K. C. Sarcopenia is an overweight or obsese patient is an adverse prognostic factor in pancreatic cancer. Clin. Cancer Res. 15, 6973–6979 (2009).
Janssen, I., Shepard, D. S., Katzmarzyk, P. T. & Roubenoff, R. The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 52, 80–85 (2004).
Beaudart, C. et al. Development of a self-administered quality of life questionnaire for sarcopenia in elderly subjects: the SarQol. Age Ageing 44, 960–966 (2015).
Brioche, T., Pagano, A. F., Py, G. & Chopard, A. Muscle wasting and aging: experimental models, fatty infiltrations and prevention. Mol. Aspects Med. 50, 56–87 (2016).
Jackson, M. J. Reactive oxygen species in sarcopenia: should we focus on excessive oxidative damage or defective redox signalling? Mol. Aspects Med. 50, 33–40 (2016).
Sousa-Victor, P. & Munoz-Canaves, P. Regenerative decline of stem cells in sarcopenia. Mol. Aspects Med. 50, 109–117 (2016).
Marzetti, E. et al. Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait speed in community-dwelling older persons: an exploratory study. PLoS ONE 7, e32829 (2012).
Sayer, A. A. et al. New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing 42, 145–150 (2013).
Piasecki, M., Ireland, A., Jones, D. A. & McPhee, J. S. Age-dependent motor unit remodelling in human limb muscles. Biogerontology 17, 485–496 (2015).
Blau, H. M., Cosgrove, B. D. & Ho, A. T. V. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 21, 854–862 (2015).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. NY Acad. Sci. 908, 244–254 (2000).
De Martinis, M., Franceschi, C., Monti, D. & Ginaldi, L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 579, 2035–2039 (2005).
Cannizzo, E. S., Clement, C. C., Sahu, R., Follo, C. & Santambrogio, L. Oxidative stress, inflamm-aging and immunosenescence. J. Proteomics 74, 2313–2323 (2011).
Ferrucci, L. et al. Serum IL-6 level and the development of disability in older persons. J. Am. Geriatr. Soc. 47, 639–646 (1999).
Wilson, D., Jackson, T., Sapey, E. & Lord, J. M. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res. Rev. 36, 1–10 (2017).
Cauley, J. A. An overview of sarcopenic obesity. J. Clin. Densitom. 18, 499–505 (2015).
Kalinkovich, A. & Livshits, G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 35, 200–221 (2016).
Villareal, D. T., Banks, M., Siener, C., Sinacore, D. R. & Klein, S. Physical frailty and body composition in obese elderly men and women. Obes. Res. 12, 913–920 (2004).
Szulc, P., Duboeuf, F., Marchand, F. & Delmas, P. Hormonal and lifestyle determinants of appendicular skeletal muscle mass in men: the MINOS study. Am. J. Clin. Nutr. 80, 496–503 (2004).
Kortebein, P. et al. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA 297, 1772–1774 (2007).
Zampieri, S. et al. Lifelong physical exercise delays age associated skeletal muscle decline. J. Gerontol. A Biol. Sci. Med. Sci. 70, 163–173 (2014).
Hinrichs, T. et al. Inverse effects of midlife occupational and leisure time physical activity on mobility limitation in old age — a 28-year prospective follow-up study. J. Am. Geriatr. Soc. 62, 812–820 (2014).
Arnold, P. & Boutmans, I. The influence of strength training on muscle activation in elderly persons: a systematic review and meta-analysis. Exp. Gerontol. 58, 58–68 (2014).
Law, T. D., Clark, L. A. & Clark, B. C. Resistance exercise to prevent and manage sarcopenia and dynapenia. Annu. Rev. Gerontol. Geriatr. 36, 205–228 (2016).
Renoud, A., Ecochard, R., Marchand, F., Chapurlat, R. & Szulc, P. Predictive parameters of accelerated muscle loss in men – MINOS study. Am. J. Med. 127, 554–561 (2014).
Curtis, E., Litwic, A., Cooper, C. & Dennison, E. Determinants of muscle and bone aging. J. Cell. Physiol. 230, 2618–2625 (2015).
Song, D. S. et al. Heavy alcohol consumption with alcoholic liver disease accelerates sarcopenia in elderly Korean males: the Korean National Health and Nutrition Examination Survey 2008–2010. PLoS ONE 11, e0163222 (2016).
Steffl, M., Bohannon, R. W., Petr, M., Kohlikova, E. & Holmerova, I. Alcohol consumption as a risk factor for sarcopenia — a meta-analysis. BMC Geriatr. 16, 99 (2016).
Rolland, Y. et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 12, 433–450 (2008).
Rizzoli, R. et al. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis. Maturitas 79, 122–132 (2014).
Short, K. R. et al. Age and aerobic exercise training effects on whole muscle body and muscle protein metabolism. Am. J. Physiol. Endocrinol. Metab. 286, E92–E101 (2004).
Volpi, E., Sheffield-Moore, M., Rasmussen, B. B. & Wolfe, R. R. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286, 1206–1212 (2001).
Rennie, M. J. et al. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin. Sci. (Lond.) 63, 519–523 (1982).
Pennings, B. et al. Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am. J. Physiol. Endocrinol. Metab. 302, E992–E999 (2012).
Denison, H. J., Cooper, C., Sayer, A. A. & Robinson, S. M. Prevention and optimal management of sarcopenia: a review of controlled exercise and nutrition interventions to improve muscle outcomes in older people. Clin. Interv. Aging 10, 859–869 (2015).
Bischoff-Ferrari, H. A. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv. Exp. Med. Biol. 810, 500–525 (2014).
Visser, M., Deeg, D. J. & Lips, P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 88, 5766–5772 (2003).
Cruz-Jentoft, A. J. et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 43, 748–759 (2014).
Reginster, J.-Y. et al. Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin. Exp. Res. 28, 47–58 (2016).
Atkinson, R. A. et al. Effects of testosterone on skeletal muscle architecture in intermediate-frail and frail elderly men. J. Gerontol. 65, 1215–1219 (2010).
Dalton, J. T. et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J. Cachexia Sarcopenia Muscle 2, 153–161 (2011).
Papanicolaou, D. A. et al. Phase IIA randomised placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J. Nutr. Health Aging 17, 533–543 (2013).
Becker, C. et al. Myostatin antibody (LY2495655) in older weak fallers: a proof of concept, randomised phase II trial. Lancet Diabetes Endocrinol. 3, 948–957 (2015).
Amato, A. A. et al. Treatment of sporadic inclusion body myositis with bimagrumab. Neurology 83, 2239–2246 (2014).
Bechir, N. et al. Mitochondrial impairment induced by postnatal ActRIIB blockade does not alter function and energy status in exercising mouse glycolytic muscle in vivo. Am. J. Physiol. Endocrinol. Metab. 310, E539–E549 (2015).
Marzetti, E. et al. Mitochondrial dysfuntion and sarcopenia of aging: from signalling pathways to clinical trials. Int. J. Biochem. Cell Biol. 45, 2288–2301 (2013).
Beaudart, C. et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 16, 170 (2016).
Martone, A. M. et al. Treating sarcopenia in older and oldest old. Curr. Pharm. Des. 21, 1715–1722 (2015).
Landi, F. et al. Sarcopenia as the biological substrate of physical frailty. Curr. Geriatr. Med. 31, 367–374 (2015).
Muscaritoli, M. et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 29, 154–159 (2010).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423 (2010).
Fielding, R. A. et al. International Working Group on Sarcopenia Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. J. Am. Med. Dir. Assoc. 12, 249–256 (2011).
Morley, J. E. et al. Sarcopenia with limited mobility: an international consensus. J. Am. Med. Dir. Assoc. 12, 403–409 (2011).
Author information
Authors and Affiliations
Contributions
E.M.D. researched data for the article and wrote the manuscript. A.A.S. and C.C. both contributed substantially to the discussion of the content, and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.C. declares that he has received consultancy fees and honoraria from Alliance for Better Bone Health, Amgen, GSK, Lilly, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB. E.M.D. declares that she has received speaker's fees from Lilly. A.S. declares no competing interests.
Rights and permissions
About this article
Cite this article
Dennison, E., Sayer, A. & Cooper, C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat Rev Rheumatol 13, 340–347 (2017). https://doi.org/10.1038/nrrheum.2017.60
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.60
This article is cited by
-
Sarcopenia in subjects with Alzheimer’s disease: prevalence and comparison of agreement between EGWSOP1, EGWSOP2, and FNIH criteria
BMC Geriatrics (2024)
-
Transcriptome-based deep learning analysis identifies drug candidates targeting protein synthesis and autophagy for the treatment of muscle wasting disorder
Experimental & Molecular Medicine (2024)
-
Association of kidney function with physical performance: the Maastricht study
Journal of Nephrology (2024)
-
Identification of a novel immune-related transcriptional regulatory network in sarcopenia
BMC Geriatrics (2023)
-
Two-year changes in body composition and future cardiovascular events: a longitudinal community-based study
Nutrition & Metabolism (2023)