Key Points
-
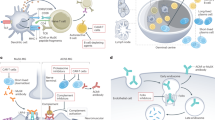
Certain autoantibodies specific for G protein-coupled receptors (GPCRs) can regulate the function of these cell surface receptors and are known as functional autoantibodies
-
Functional autoantibodies targeting different GPCRs might influence the outcome of autoimmune diseases
-
Growing evidence points to a pathological role of autoantibodies against endothelin receptor type A (ETAR) and type 1 angiotensin II receptor (AT1R)
-
Anti-AT1R and anti-ETAR autoantibodies can trigger the same signalling pathways as those activated by endogenous ligands, ultimately regulating the same cellular events
-
The concentration of anti-GPCR autoantibodies in the serum of healthy individuals is usually lower than in that of patients with autoimmune disorders
Abstract
G protein-coupled receptors (GPCRs) comprise the largest and most diverse family of integral membrane proteins that participate in different physiological processes such as the regulation of the nervous and immune systems. Besides the endogenous ligands of GPCRs, functional autoantibodies are also able to bind GPCRs to trigger or block intracellular signalling pathways, resulting in agonistic or antagonistic effects, respectively. In this Review, the effects of functional GPCR-targeting autoantibodies on the pathogenesis of autoimmune diseases, including rheumatic diseases, are discussed. Autoantibodies targeting β1 and β2 adrenergic receptors, which are expressed by cardiac and airway smooth muscle cells, respectively, have an important role in the development of asthma and cardiovascular diseases. In addition, high levels of autoantibodies against the muscarinic acetylcholine receptor M3 as well as those targeting endothelin receptor type A and type 1 angiotensin II receptor have several implications in the pathogenesis of rheumatic diseases such as Sjögren syndrome and systemic sclerosis. Expanding the knowledge of the pathophysiological roles of autoantibodies against GPCRs will shed light on the biology of these receptors and open avenues for new therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Venkatakrishnan, A. J. et al. Molecular signatures of G-protein-coupled receptors. Nature 494, 185–194 (2013).
Flock, T. et al. Selectivity determinants of GPCR-G-protein binding. Nature 545, 317–322 (2017).
Nomiyama, H., Osada, N. & Yoshie, O. A family tree of vertebrate chemokine receptors for a unified nomenclature. Dev. Comp. Immunol. 35, 705–715 (2011).
Bryant, V. L. & Slade, C. A. Chemokines, their receptors and human disease: the good, the bad and the itchy. Immunol. Cell Biol. 93, 364–371 (2015).
Enghard, P. et al. CXCR3+CD4+ T cells are enriched in inflamed kidneys and urine and provide a new biomarker for acute nephritis flares in systemic lupus erythematosus patients. Arthritis Rheum. 60, 199–206 (2009).
Ruth, J. H. et al. Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis Rheum. 44, 2750–2760 (2001).
Balashov, K. E., Rottman, J. B., Weiner, H. L. & Hancock, W. W. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc. Natl Acad. Sci. USA 96, 6873–6878 (1999).
Donath, J. K. & Landsteiner, K. Uber paroxysmale haemoglobinurie. Munch. Med. Wochenschr. 51, 1590–1593 (1904).
Ludwig, R. J. et al. Mechanisms of autoantibody-induced pathology. Front. Immunol. 8, 603 (2017).
Herda, L. R., Felix, S. B. & Boege, F. Drug-like actions of autoantibodies against receptors of the autonomous nervous system and their impact on human heart function. Br. J. Pharmacol. 166, 847–857 (2012).
Gleicher, N., Barad, D. & Weghofer, A. Functional autoantibodies, a new paradigm in autoimmunity? Autoimmun. Rev. 7, 42–45 (2007).
Riemekasten, G. et al. Involvement of functional autoantibodies against vascular receptors in systemic sclerosis. Ann. Rheum. Dis. 70, 530–536 (2011).
Gabrielli, A., Moroncini, G., Svegliati, S. & Avvedimento, E. V. Autoantibodies against the platelet-derived growth factor receptor in scleroderma: comment on the articles by Clessen et al and Loizos et al. Arthritis Rheum. 60, 3521–3522 (2009).
Venter, J. C., Fraser, C. M. & Harrison, L. C. Autoantibodies to beta 2-adrenergic receptors: a possible cause of adrenergic hyporesponsiveness in allergic rhinitis and asthma. Science 207, 1361–1363 (1980).
Gleicher, N., Harlow, L. & Zilberstein, M. Regulatory effect of antiphospholipid antibodies on signal transduction: a possible model for autoantibody-induced reproductive failure. Am. J. Obstet. Gynecol. 167, 637–642 (1992).
Lunardi, C. et al. Antibodies against human cytomegalovirus in the pathogenesis of systemic sclerosis: a gene array approach. PLoS Med. 3, e2 (2005).
Wolf, S. I., Howat, S., Abraham, D. J., Pearson, J. D. & Lawson, C. Agonistic anti-ICAM-1 antibodies in scleroderma: activation of endothelial pro-inflammatory cascades. Vascul. Pharmacol. 59, 19–26 (2013).
Cabral-Marques, O. & Riemekasten, G. Vascular hypothesis revisited: role of stimulating antibodies against angiotensin and endothelin receptors in the pathogenesis of systemic sclerosis. Autoimmun. Rev. 15, 690–694 (2016).
Dragun, D. et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N. Engl. J. Med. 352, 558–569 (2005).
Baroni, S. et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N. Engl. J. Med. 354, 2667–2676 (2006).
Matsui, S. et al. Dilated cardiomyopathy defines serum autoantibodies against G-protein-coupled cardiovascular receptors. Autoimmunity 21, 85–88 (1995).
Jo, M. & Jung, S. T. Engineering therapeutic antibodies targeting G-protein–coupled receptors. Exp. Mol. Med. 48, e207 (2016).
Gether, U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 21, 90–113 (2000).
Johnson, M. Molecular mechanisms of β2-adrenergic receptor function, response, and regulation. J. Allergy Clin. Immunol. 117, 18–24 (2006).
McCorry, L. K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 71, 78 (2007).
McGraw, D. W. & Liggett, S. B. Molecular mechanisms of beta2-adrenergic receptor function and regulation. Proc. Am. Thorac. Soc. 2, 292–296 (2005).
Strosberg, A. D. Structure, function, and regulation of adrenergic receptors. Protein Sci. 2, 1198–1209 (1993).
Lorton, D. & Bellinger, D. L. Molecular mechanisms underlying β-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells. Int. J. Mol. Sci. 16, 5635–5665 (2015).
Turki, J. & Liggett, S. B. Receptor-specific functional properties of beta 2-adrenergic receptor autoantibodies in asthma. Am. J. Respir. Cell Mol. Biol. 12, 531–539 (1995).
Loebel, M. et al. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with chronic fatigue syndrome. Brain Behav. Immun. 52, 32–39 (2016).
Kohr, D. et al. Autoimmunity against the β2 adrenergic receptor and muscarinic-2 receptor in complex regional pain syndrome. Pain 152, 2690–2700 (2011).
Segovia, M., Ganzinelli, S., Reina, S., Borda, E. & Sterin-Borda, L. Role of anti-β1 adrenergic antibodies from patients with periodontitis in cardiac dysfunction. J. Oral Pathol. Med. 41, 242–248 (2012).
Zuo, L. et al. Long-term active immunization with a synthetic peptide corresponding to the second extracellular loop of β1-adrenoceptor induces both morphological and functional cardiomyopathic changes in rats. Int. J. Cardiol. 149, 89–94 (2011).
Borda, E. et al. A circulating IgG in Chagas' disease which binds to beta-adrenoceptors of myocardium and modulates their activity. Clin. Exp. Immunol. 57, 679–686 (1984).
Limas, C. J., Goldenberg, I. F. & Limas, C. Autoantibodies against beta-adrenoceptors in human idiopathic dilated cardiomyopathy. Circ. Res. 64, 97–103 (1989).
Tate, K. et al. Epitope analysis of T− and B-cell response against the human beta 1-adrenoceptor. Biochimie 76, 159–164 (1994).
Tutor, A. S., Penela, P. & Mayor, F. Anti-beta1-adrenergic receptor autoantibodies are potent stimulators of the ERK1/2 pathway in cardiac cells. Cardiovasc. Res. 76, 51–60 (2007).
Magnusson, Y., Wallukat, G., Guillet, J. G., Hjalmarson, A. & Hoebeke, J. Functional analysis of rabbit anti-peptide antibodies which mimic autoantibodies against the beta 1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J. Autoimmun. 4, 893–905 (1991).
Williams, L. T., Mullikin, D. & Lefkowitz, R. J. Magnesium dependence of agonist binding to adenylate cyclase-coupled hormone receptors. J. Biol. Chem. 253, 2984–2989 (1978).
Gong, Y. et al. Autoantibodies against β1-adrenoceptor induce blood glucose enhancement and insulin insufficient via T lymphocytes. Immunol. Res. 64, 584–593 (2016).
Scanzano, A. & Cosentino, M. Adrenergic regulation of innate immunity: a review. Front. Pharmacol. 6, 171 (2015).
Du, Y. et al. β1-adrenergic receptor autoantibodies from heart failure patients enhanced TNF-α secretion in RAW264.7 macrophages in a largely PKA-dependent fashion. J. Cell. Biochem. 113, 3218–3228 (2012).
Du, Y. et al. β1-adrenoceptor autoantibodies from DCM patients enhance the proliferation of T lymphocytes through the β1-AR/cAMP/PKA and p38 MAPK pathways. PLoS ONE 7, e52911 (2012).
Schulze-Koops, H. & Kalden, J. R. The balance of Th1/Th2 cytokines in rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 15, 677–691 (2001).
Kalliolias, G. D. & Ivashkiv, L. B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 12, 49–62 (2015).
Kruse, A. C. et al. Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat. Rev. Drug Discov. 13, 549–560 (2014).
Wessler, I. & Kirkpatrick, C. J. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol. 154, 1558–1571 (2008).
Razani-Boroujerdi, S. et al. Role of muscarinic receptors in the regulation of immune and inflammatory responses. J. Neuroimmunol. 194, 83–88 (2008).
Kovács, L. et al. Clinical associations of autoantibodies to human muscarinic acetylcholine receptor 3213–3228 in primary Sjögren's syndrome. Rheumatology 44, 1021–1025 (2005).
Tsuboi, H. et al. New epitopes and function of anti-M3 muscarinic acetylcholine receptor antibodies in patients with Sjögren's syndrome. Clin. Exp. Immunol. 162, 53–61 (2010).
Park, K., Haberberger, R. V., Gordon, T. P. & Jackson, M. W. Antibodies interfering with the type 3 muscarinic receptor pathway inhibit gastrointestinal motility and cholinergic neurotransmission in Sjögren's syndrome. Arthritis Rheum. 63, 1426–1434 (2011).
Takeuchi, T., Tanaka, K., Nakajima, H., Matsui, M. & Azuma, Y. T. M2 and M3 muscarinic receptors are involved in enteric nerve-mediated contraction of the mouse ileum: findings obtained with muscarinic-receptor knockout mouse. Am. J. Physiol. Gastrointest. Liver Physiol. 292, G154–G164 (2007).
Wess, J., Eglen, R. M. & Gautam, D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat. Rev. Drug Discov. 6, 721–733 (2007).
Unno, T. et al. Roles of M2 and M3 muscarinic receptors in cholinergic nerve-induced contractions in mouse ileum studied with receptor knockout mice. Br. J. Pharmacol. 149, 1022–1030 (2009).
Kuriyama, H., Kitamura, K., Itoh, T. & Inoue, R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol. Rev. 78, 811–920 (1998).
Scarselli, M., Li, B., Kim, S. K. & Wess, J. Multiple residues in the second extracellular loop are critical for M3 muscarinic acetylcholine receptor activation. J. Biol. Chem. 282, 7385–7396 (2006).
Preuss, B., Tunaru, S., Henes, J., Offermanns, S. & Klein, R. A novel luminescence-based method for the detection of functionally active antibodies to muscarinic acetylcholine receptors of the M3 type (mAchR3) in patients' sera. Clin. Exp. Immunol. 177, 179–189 (2014).
Watson, E. L. et al. Identification of muscarinic receptor subtypes in mouse parotid gland. Am. J. Physiol. 271, C905–C913 (1996).
Goldblatt, F., Gordon, T. P. & Waterman, S. A. Antibody-mediated gastrointestinal dysmotility in scleroderma. Gastroenterology 123, 1144–1150 (2002).
Kawaguchi, Y. et al. Muscarinic-3 acetylcholine receptor autoantibody in patients with systemic sclerosis: contribution to severe gastrointestinal tract dysmotility. Ann. Rheum. Dis. 68, 710–714 (2009).
Shreiner, A. B., Murray, C., Denton, C. & Khanna, D. Gastrointestinal manifestations of systemic sclerosis. J. Scleroderma Relat. Disord. 1, 247–256 (2016).
Günther, J. et al. Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients. Arthritis Res. Ther. 16, R65 (2014).
Kill, A. et al. Autoantibodies to angiotensin and endothelin receptors in systemic sclerosis induce cellular and systemic events associated with disease pathogenesis. Arthritis Res. Ther. 16, R29 (2014).
Yamin, R. & Morgan, K. G. Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell. J. Physiol. 590, 4145–4154 (2012).
Ishida, T., Ishida, M., Suero, J., Takahashi, M. & Berk, B. C. Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J. Clin. Invest. 103, 789–797 (1999).
Schappi, J. M., Krbanjevic, A. & Rasenick, M. M. Tubulin, actin and heterotrimeric G proteins: coordination of signaling and structure. Biochim. Biophys. Acta 1838, 674–681 (2014).
Tang, D. D. & Anfinogenova, Y. Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J. Cardiovasc. Pharmacol. Ther. 13, 130–140 (2008).
Drawnel, F. M., Archer, C. R. & Roderick, H. L. The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. Br. J. Pharmacol. 168, 296–317 (2013).
de Frutos, S., Ramiro Diaz, J. M., Nitta, C. H., Sherpa, M. L. & Gonzalez Bosc, L. V. Endothelin-1 contributes to increased NFATc3 activation by chronic hypoxia in pulmonary arteries. Am. J. Cell Physiol. 301, C441–C450 (2011).
Kawaguchi, Y. et al. Angiotensin II in the lesional skin of systemic sclerosis patients contributes to tissue fibrosis via angiotensin II type 1 receptors. Arthritis Rheum. 50, 216–226 (2004).
Kawaguchi, Y. et al. Increased endothelin-1 production in fibroblasts derived from patients with systemic sclerosis. Ann. Rheum. Dis. 53, 506–510 (1994).
Ruiz-Ortega, M. et al. Angiotensin II activates nuclear transcription factor kappaB through AT(1) and AT(2) in vascular smooth muscle cells: molecular mechanisms. Circ. Res. 86, 1266–1272 (2000).
Harrison, J. G., Sugden, P. H. & Clerk, A. Endothelin-1 promotes phosphorylation of CREB transcription factor in primary cultures of neonatal rat cardiac myocytes: implications for the regulation of c-jun expression. Biochim. Biophys. Acta 1644, 17–25 (2004).
Gallois, C. et al. Role of NF-kappaB in the antiproliferative effect of endothelin-1 and tumor necrosis factor-alpha in human hepatic stellate cells. Involvement of cyclooxygenase-2. J. Biol. Chem. 273, 23183–23190 (1998).
Guo, L. et al. Anti-endothelin receptor type A autoantibodies in systemic lupus erythematosus-associated pulmonary arterial hypertension. Arthritis Rheumatol. 67, 2394–2402 (2015).
Xiong, J., Liang, Y., Yang, H., Zhu, F. & Wang, Y. The role of angiotensin II type 1 receptor-activating antibodies in patients with lupus nephritis. Int. J. Clin. Pract. 67, 1066–1067 (2013).
Elferink, J. G. & de Koster, B. M. The stimulation of human neutrophil migration by angiotensin IL: its dependence on Ca2+ and the involvement of cyclic GMP. Br. J. Pharmacol. 121, 643–648 (1997).
József, L., Khreiss, T., Fournier, A., Chan, J. S. D. & Filep, J. G. Extracellular signal-regulated kinase plays an essential role in endothelin-1-induced homotypic adhesion of human neutrophil granulocytes. Br. J. Pharmacol. 135, 1167–1174 (2002).
Zouki, C., Baron, C., Fournier, A. & Filep, J. G. Endothelin-1 enhances neutrophil adhesion to human coronary artery endothelial cells: role of ET(A) receptors and platelet-activating factor. Br. J. Pharmacol. 127, 969–979 (1999).
Achmad, T. H. & Rao, G. S. Chemotaxis of human blood monocytes toward endothelin-1 and the influence of calcium channel blockers. Biochem. Biophys. Res. Commun. 189, 994–1000 (1992).
Kintscher, U. et al. Angiotensin II induces migration and Pyk2/paxillin phosphorylation of human monocytes. Hypertension 37, 587–593 (2001).
Kranzhöfer, R. et al. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 19, 1623–1629 (1999).
Browatzki, M., Schmidt, J., Kübler, W. & Kranzhöfer, R. Endothelin-1 induces interleukin-6 release via activation of the transcription factor NF-kappaB in human vascular smooth muscle cells. Basic Res. Cardiol. 95, 98–105 (2000).
Elferink, J. G. & De Koster, B. M. The involvement of protein kinase G in stimulation of neutrophil migration by endothelins. Eur. J. Pharmacol. 350, 285–291 (1998).
Ruiz-Ortega, M., Lorenzo, O. & Egido, J. Angiotensin III increases MCP-1 and activates NF-κB and AP-1 in cultured mesangial and mononuclear cells. Kidney Int. 57, 2285–2298 (2000).
Ruiz-Ortega, M. et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int. 62, S12–S22 (2002).
Sampaio, A. L. F., Rae, G. A. & Henriques, M. G. Effects of endothelin ETA receptor antagonism on granulocyte and lymphocyte accumulation in LPS-induced inflammation. J. Leukoc. Biol. 76, 210–216 (2004).
Hofman, F. M. et al. Endothelin-1 induces production of the neutrophil chemotactic factor interleukin-8 by human brain-derived endothelial cells. Blood 92, 3064–3072 (1998).
Koyama, Y. et al. Different actions of endothelin-1 on chemokine production in rat cultured astrocytes: reduction of CX3CL1/fractalkine and an increase in CCL2/MCP-1 and CXCL1/CINC-1. J. Neuroinflamm. 10, 51 (2013).
Kill, A. & Riemekasten, G. Functional autoantibodies in systemic sclerosis pathogenesis. Curr. Rheumatol. Rep. 17, 34 (2015).
Rabquer, B. J. et al. The proadhesive phenotype of systemic sclerosis skin promotes myeloid cell adhesion via ICAM-1 and VCAM-1. Rheumatology (Oxford) 48, 734–740 (2009).
Krohn, S., Garin, A., Gabay, C. & Proudfoot, A. E. I. The activity of CCL18 is principally mediated through interaction with glycosaminoglycans. Front. Immunol. 4, 193 (2013).
Islam, S. A., Ling, M. F., Leung, J., Shreffler, W. G. & Luster, A. D. Identification of human CCR8 as a CCL18 receptor. J. Exp. Med. 210, 1889–1898 (2013).
Rademacher, J. et al. Monocytic angiotensin and endothelin receptor imbalance modulate secretion of the profibrotic chemokine ligand 18. J. Rheumatol. 43, 587–591 (2016).
Schupp, J. et al. Serum CCL18 is predictive for lung disease progression and mortality in systemic sclerosis. Eur. Respir. J. 43, 1530–1532 (2014).
Shi-wen, X. et al. Focal adhesion kinase and reactive oxygen species contribute to the persistent fibrotic phenotype of lesional scleroderma fibroblasts. Rheumatology 51, 2146–2154 (2012).
Becker, M. O. et al. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am. J. Respir. Crit. Care Med. 190, 808–817 (2014).
Tomar, N., Gupta, N. & Goswami, R. Calcium-sensing receptor autoantibodies and idiopathic hypoparathyroidism. J. Clin. Endocrinol. Metab. 98, 3884–3891 (2013).
Kifor, O. et al. Activating antibodies to the calcium-sensing receptor in two patients with autoimmune hypoparathyroidism. J. Clin. Endocrinol. Metab. 89, 548–556 (2004).
Minich, W. B., Lenzner, C. & Morgenthaler, N. G. Antibodies to TSH-receptor in thyroid autoimmune disease interact with monoclonal antibodies whose epitopes are broadly distributed on the receptor. Clin. Exp. Immunol. 136, 129–136 (2004).
Riccardi, D. & Valenti, G. Localization and function of the renal calcium-sensing receptor. Nat. Rev. Nephrol. 12, 414–425 (2016).
Harris, H. E., Kemp, E. H., Brown, E. M., Weetman, A. P. & Swaminathan, K. First report of anti-calcium-sensing receptor antibodies in a patient with Sjogren's syndrome and primary hypoparathyroidism. Rheumatology (Oxford) 50, 1173–1175 (2011).
Dutta, D., Das, R. N., Ghosh, S., Mukhopadhyay, S. & Chowdhury, S. Idiopathic hypoparathyroidism and systemic sclerosis: an association likely missed. Indian J. Endocrinol. Metab. 16, S396–398 (2012).
Attout, H. et al. Hypoparathyroidism in systemic lupus erythematosus. Joint Bone Spine 74, 282–284 (2007).
Biró, E. et al. Association of systemic and thyroid autoimmune diseases. Clin. Rheumatol. 25, 240–245 (2006).
Marker, M., Derfler, K., Monshi, B. & Rappersberger, K. Successful immunoapheresis of bullous autoimmune diseases: pemphigus vulgaris and pemphigoid gestationis. J. Dtsch. Dermatol. Ges. 9, 27–31 (2011).
Riemekasten, G. & Cabral-Marques, O. Antibodies against angiotensin II type 1 receptor (AT1R) and endothelin receptor type A (ETAR) in systemic sclerosis (SSc)-response. Autoimmun. Rev. 15, 935 (2016).
Stummvoll, G. Immunoadsorption (IAS) for systemic lupus erythematosus. Lupus 20, 115–119 (2011).
Wallukat, G. et al. Aptamer BC007 for neutralization of pathogenic autoantibodies directed against G-protein coupled receptors: a vision of future treatment of patients with cardiomyopathies and positivity for those autoantibodies. Atherosclerosis 244, 44–47 (2016).
Rajagopal, S., Rajagopal, K. & Lefkowitz, R. J. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nat. Rev. Drug Discov. 9, 373–386 (2010).
Boerrigter, G., Soergel, D. G., Violin, J. D., Lark, M. W. & Burnett, J. C. TRV120027, a novel β-arrestin biased ligand at the angiotensin II type I receptor, unloads the heart and maintains renal function when added to furosemide in experimental heart failure. Circ. Heart Fail. 5, 627–634 (2012).
Pang, P. S. et al. Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: a randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF). Eur. Heart J. http://dx.doi.org/10.1093/eurheartj/ehx196 (2017).
Soergel, D. G., Subach, R. A., Cowan, C. L., Violin, J. D. & Lark, M. W. First clinical experience with TRV027: pharmacokinetics and pharmacodynamics in healthy volunteers. J. Clin. Pharmacol. 53, 892–899 (2013).
Overington, J. P., Al-Lazikani, B. & Hopkins, A. L. How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 (2006).
Newman, J. H., Kar, S. & Kirkpatrick, P. Ambrisentan. Nat. Rev. Drug Discov. 6, 697–698 (2007).
Denton, C. P. et al. Long-term effects of bosentan on quality of life, survival, safety and tolerability in pulmonary arterial hypertension related to connective tissue diseases. Ann. Rheum. Dis. 67, 1222–1228 (2008).
Lopez-Ovejero, J. A. et al. Reversal of vascular and renal crises of scleroderma by oral angiotensin-converting-enzyme blockade. N. Engl. J. Med. 300, 1417–1419 (1979).
Liu, H. R., Zhao, R. R., Zhi, J. M., Wu, B. W. & Fu, M. L. Screening of serum autoantibodies to cardiac beta1-adrenoceptors and M2-muscarinic acetylcholine receptors in 408 healthy subjects of varying ages. Autoimmunity 29, 43–51 (1999).
Sitaru, C., Mihai, S. & Zillikens, D. The relevance of the IgG subclass of autoantibodies for blister induction in autoimmune bullous skin diseases. Arch. Dermatol. Res. 299, 1–8 (2007).
Roescher, N., Kingman, A., Shirota, Y., Chiorini, J. A. & Illei, G. G. Peptide-based ELISAs are not sensitive and specific enough to detect muscarinic receptor type 3 autoantibodies in serum from patients with Sjogren's syndrome. Ann. Rheum. Dis. 70, 235–236 (2011).
Park, K., Park, S. & Jackson, M. W. The inhibitory effects of antimuscarinic autoantibodies in the sera of primary Sjogren syndrome patients on the gastrointestinal motility. Mol. Immunol. 56, 583–587 (2013).
Acknowledgements
We thank Dr Lena F Schimke-Marques and Dr. Antje Müller from the Department of Rheumatology, University of Lübeck for her critical reading of the manuscript. We would also like to acknowledge the support and funding from the intramural grant of the University of Lübeck, German Systemic Sclerosis Network (DNSS), DFG grant RI 1056-11-1/2, Actelion Pharmaceutical GmbH Germany, GSK, CellTrend, Scleroderma Foundation, and Mirjam Lichy Foundation.
Author information
Authors and Affiliations
Contributions
All the authors researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Cabral-Marques, O., Riemekasten, G. Functional autoantibodies targeting G protein-coupled receptors in rheumatic diseases. Nat Rev Rheumatol 13, 648–656 (2017). https://doi.org/10.1038/nrrheum.2017.134
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2017.134
This article is cited by
-
Palpitation in women with silicone breast implants: association with autoantibodies against autonomic nervous system
Immunologic Research (2024)
-
Severe COVID-19 patients exhibit elevated levels of autoantibodies targeting cardiolipin and platelet glycoprotein with age: a systems biology approach
npj Aging (2023)
-
Transcutaneous vagus nerve stimulation attenuates autoantibody-mediated cardiovagal dysfunction and inflammation in a rabbit model of postural tachycardia syndrome
Journal of Interventional Cardiac Electrophysiology (2022)
-
Study of Plasma Anti-CD26 Autoantibody Levels in a Cohort of Treatment-Naïve Early Arthritis Patients
Archivum Immunologiae et Therapiae Experimentalis (2022)
-
Autoantibodies targeting GPCRs and RAS-related molecules associate with COVID-19 severity
Nature Communications (2022)