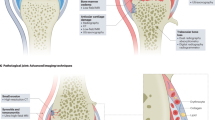
Key Points
-
Mass spectrometry imaging (MSI) enables the determination of the relative abundance and spatial distribution of biomolecules in tissue sections without labelling or staining
-
Studies of joint samples have demonstrated that MSI can provide complementary information to histology and histochemistry for rheumatic disorders
-
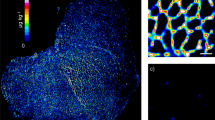
MSI has revealed the identity and distribution of several peptides, lipids and chemical elements in cartilage, synovium and bone from patients with rheumatic diseases
-
Although MSI is currently mainly used in research, this technology might soon be introduced into clinical practice owing to rapid technological improvements
Abstract
Mass spectrometry imaging (MSI) is used to determine the relative abundance and spatial distribution of biomolecules such as peptides, proteins, lipids and other organic compounds in tissue sections by their molecular masses. This technique provides a sensitive and label-free approach for high-resolution imaging, and is currently used in an increasing number of biomedical applications such as biomarker discovery, tissue classification and drug monitoring. Owing to technological advances in the past 5 years in diverse MSI strategies, this technology is expected to become a standard tool in clinical practice and provides information complementary to that obtained using existing methods. Given that MSI is able to extract mass-spectral signatures from pathological tissue samples, this technique provides a novel platform to study joint-related tissues affected by rheumatic diseases. In rheumatology, MSI has been performed on articular cartilage, synovium and bone to increase the understanding of articular destruction and to characterize diagnostic and prognostic biomarkers for osteoarthritis, rheumatoid arthritis and osteoporosis. In this Review, we provide an overview of MSI technology and of the studies in which joint tissues have been analysed by use of this methodology. This approach might increase knowledge of rheumatic pathologies and ultimately prompt the development of targeted strategies for their management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Chaurand, P. Imaging mass spectrometry of thin tissue sections: a decade of collective efforts. J. Proteom. 75, 4883–4892 (2012).
Gode, D. & Volmer, D. A. Lipid imaging by mass spectrometry - a review. Analyst 138, 1289–1315 (2013).
Ferguson, C. N., Fowler, J. W., Waxer, J. F., Gatti, R. A. & Loo, J. A. Mass spectrometry-based tissue imaging of small molecules. Adv. Exp. Med. Biol. 806, 283–299 (2014).
Rial-Otero, R. et al. Sonoreactor-based technology for fast high-throughput proteolytic digestion of proteins. J. Proteome Res. 6, 909–912 (2007).
Gagnon, H. et al. Targeted mass spectrometry imaging: specific targeting mass spectrometry imaging technologies from history to perspective. Prog. Histochem. Cytochem. 47, 133–174 (2012).
Wu, C., Dill, A. L., Eberlin, L. S., Cooks, R. G. & Ifa, D. R. Mass spectrometry imaging under ambient conditions. Mass Spectrom. Rev. 32, 218–243 (2013).
McDonnell, L. A. & Heeren, R. M. Imaging mass spectrometry. Mass Spectrom. Rev. 26, 606–643 (2007).
Svensson, M. et al. Heat stabilization of the tissue proteome: a new technology for improved proteomics. J. Proteome Res. 8, 974–981 (2009).
Groseclose, M. R., Massion, P. P., Chaurand, P. & Caprioli, R. M. High-throughput proteomic analysis of formalin-fixed paraffin-embedded tissue microarrays using MALDI imaging mass spectrometry. Proteomics 8, 3715–3724 (2008).
Casadonte, R. & Caprioli, R. M. Proteomic analysis of formalin-fixed paraffin-embedded tissue by MALDI imaging mass spectrometry. Nat. Protoc. 6, 1695–1709 (2011).
Schwartz, S. A., Reyzer, M. L. & Caprioli, R. M. Direct tissue analysis using matrix-assisted laser desorption/ionization mass spectrometry: practical aspects of sample preparation. J. Mass Spectrom. 38, 699–708 (2003).
Crecelius, A. C. et al. Three-dimensional visualization of protein expression in mouse brain structures using imaging mass spectrometry. J. Am. Soc. Mass Spectrom. 16, 1093–1099 (2005).
Chaurand, P. et al. Integrating histology and imaging mass spectrometry. Anal. Chem. 76, 1145–1155 (2004).
Chughtai, K., Jiang, L., Greenwood, T. R., Glunde, K. & Heeren, R. M. Mass spectrometry images acylcarnitines, phosphatidylcholines, and sphingomyelin in MDA-MB-231 breast tumor models. J. Lipid Res. 54, 333–344 (2013).
Deutskens, F., Yang, J. & Caprioli, R. M. High spatial resolution imaging mass spectrometry and classical histology on a single tissue section. J. Mass Spectrom. 46, 568–571 (2011).
Seeley, E. H., Oppenheimer, S. R., Mi, D., Chaurand, P. & Caprioli, R. M. Enhancement of protein sensitivity for MALDI imaging mass spectrometry after chemical treatment of tissue sections. J. Am. Soc. Mass Spectrom. 19, 1069–1077 (2008).
Chacon, A. et al. On-tissue chemical derivatization of 3-methoxysalicylamine for MALDI-imaging mass spectrometry. J. Mass Spectrom. 46, 840–846 (2011).
Kaletas, B. K. et al. Sample preparation issues for tissue imaging by imaging MS. Proteomics 9, 2622–2633 (2009).
Svara, F. N., Kiss, A., Jaskolla, T. W., Karas, M. & Heeren, R. M. High-reactivity matrices increase the sensitivity of matrix enhanced secondary ion mass spectrometry. Anal. Chem. 83, 8308–8313 (2011).
Pan, C. et al. Recent developments in methods and technology for analysis of biological samples by MALDI-TOF-MS. Anal. Bioanal. Chem. 387, 193–204 (2007).
Tholey, A. & Heinzle, E. Ionic (liquid) matrices for matrix-assisted laser desorption/ionization mass spectrometry-applications and perspectives. Anal. Bioanal. Chem. 386, 24–37 (2006).
Passarelli, M. K. & Winograd, N. Lipid imaging with time-of-flight secondary ion mass spectrometry (ToF-SIMS). Biochim. Biophys. Acta 1811, 976–990 (2011).
Lockyer, N. P. Secondary ion mass spectrometry imaging of biological cells and tissues. Methods Mol. Biol. 1117, 707–732 (2014).
Altelaar, A. F. & Piersma, S. R. Cellular imaging using matrix-enhanced and metal-assisted SIMS. Methods Mol. Biol. 656, 197–208 (2010).
Northen, T. R. et al. Clathrate nanostructures for mass spectrometry. Nature 449, 1033–1036 (2007).
O'Brien, P. J. et al. Monitoring metabolic responses to chemotherapy in single cells and tumors using nanostructure-initiator mass spectrometry (NIMS) imaging. Cancer Metab. 1, 4 (2013).
Kurczy, M. E., Northen, T. R., Trauger, S. A. & Siuzdak, G. Nanostructure imaging mass spectrometry: the role of fluorocarbons in metabolite analysis and yoctomole level sensitivity. Methods Mol. Biol. 1203, 141–149 (2015).
Lee, d. Y. et al. Resolving brain regions using nanostructure initiator mass spectrometry imaging of phospholipids. Integr. Biol. 4, 693–699 (2012).
Sturm, R. M., Greer, T., Chen, R., Hensen, B. & Li, L. Comparison of NIMS and MALDI platforms for neuropeptide and lipid mass spectrometric imaging in C. borealis brain tissue. Anal. Methods 5, 1623–1628 (2013).
Ifa, D. R., Gumaelius, L. M., Eberlin, L. S., Manicke, N. E. & Cooks, R. G. Forensic analysis of inks by imaging desorption electrospray ionization (DESI) mass spectrometry. Analyst 132, 461–467 (2007).
Campbell, D. I., Ferreira, C. R., Eberlin, L. S. & Cooks, R. G. Improved spatial resolution in the imaging of biological tissue using desorption electrospray ionization. Anal. Bioanal. Chem. 404, 389–398 (2012).
Wu, C., Ifa, D. R., Manicke, N. E. & Cooks, R. G. Molecular imaging of adrenal gland by desorption electrospray ionization mass spectrometry. Analyst 135, 28–32 (2010).
Wiseman, J. M. et al. Desorption electrospray ionization mass spectrometry: Imaging drugs and metabolites in tissues. Proc. Natl Acad. Sci. USA 105, 18120–18125 (2008).
Eberlin, L. S., Ifa, D. R., Wu, C. & Cooks, R. G. Three-dimensional vizualization of mouse brain by lipid analysis using ambient ionization mass spectrometry. Angew. Chem. Int. Ed. Engl. 49, 873–876 (2010).
Eberlin, L. S. et al. Classifying human brain tumors by lipid imaging with mass spectrometry. Cancer Res. 72, 645–654 (2012).
Jones, E. A., Deininger, S. O., Hogendoorn, P. C., Deelder, A. M. & McDonnell, L. A. Imaging mass spectrometry statistical analysis. J. Proteom. 75, 4962–4989 (2012).
McCombie, G., Staab, D., Stoeckli, M. & Knochenmuss, R. Spatial and spectral correlations in MALDI mass spectrometry images by clustering and multivariate analysis. Anal. Chem. 77, 6118–6124 (2005).
Klerk, L. A. et al. TOF-secondary ion mass spectrometry imaging of polymeric scaffolds with surrounding tissue after in vivo implantation. Anal. Chem. 82, 4337–4343 (2010).
Rocha, B. et al. Characterization of lipidic markers of chondrogenic differentiation using mass spectrometry imaging. Proteomics 15, 702–713 (2015).
McDonnell, L. A. et al. Peptide and protein imaging mass spectrometry in cancer research. J. Proteom. 73, 1921–1944 (2010).
Djidja, M. C. et al. Novel molecular tumour classification using MALDI-mass spectrometry imaging of tissue micro-array. Anal. Bioanal. Chem. 397, 587–601 (2010).
Cillero-Pastor, B., Eijkel, G. B., Kiss, A., Blanco, F. J. & Heeren, R. M. Matrix-assisted laser desorption ionization-imaging mass spectrometry: a new methodology to study human osteoarthritic cartilage. Arthritis Rheum. 65, 710–720 (2013).
Yasugi, E. & Watanabe, K. [LIPIDBANK for Web, the newly developed lipid database]. Tanpakushitsu Kakusan Koso 47, 837–841 (2002).
Taguchi, R., Nishijima, M. & Shimizu, T. Basic analytical systems for lipidomics by mass spectrometry in Japan. Methods Enzymol. 432, 185–211 (2007).
Fahy, E., Sud, M., Cotter, D. & Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 35, W606–612 (2007).
Sud, M. et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 35, D527–D532 (2007).
Wishart, D. S. et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 41, D801–D807 (2013).
Kind, T. et al. LipidBlast in silico tandem mass spectrometry database for lipid identification. Nat. Methods 10, 755–758 (2013).
Smith, C. A. et al. METLIN: a metabolite mass spectral database. Ther. Drug Monit. 27, 747–751 (2005).
Kanehisa, M. The KEGG database. Novartis Found. Symp. 247, 91–101 (2002).
McDonnell, L. A., Walch, A., Stoeckli, M. & Corthals, G. L. MSiMass list: a public database of identifications for protein MALDI MS imaging. J. Proteome Res. 13, 1138–1142 (2014).
Maier, S. K. et al. Comprehensive identification of proteins from MALDI imaging. Mol. Cell Proteom. 12, 2901–2910 (2013).
Rebours, V. et al. In situ proteomic analysis by MALDI imaging identifies ubiquitin and thymosin-β4 as markers of malignant intraductal pancreatic mucinous neoplasms. Pancreatology 14, 117–124 (2014).
Cillero-Pastor, B., Eijkel, G., Kiss, A., Blanco, F. J. & Heeren, R. M. Time-of-flight secondary ion mass spectrometry-based molecular distribution distinguishing healthy and osteoarthritic human cartilage. Anal. Chem. 84, 8909–8916 (2012).
Goldring, M. B. & Goldring, S. R. Osteoarthritis. J. Cell. Physiol. 213, 626–634 (2007).
Sellam, J. & Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 (2010).
Goldring, M. B. & Berenbaum, F. The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin. Orthop. Relat. Res. 427 (Suppl), S37–S46 (2004).
Peffers, M. J., Cillero-Pastor, B., Eijkel, G. B., Clegg, P. D. & Heeren, R. M. Matrix assisted laser desorption ionization mass spectrometry imaging identifies markers of ageing and osteoarthritic cartilage. Arthritis Res. Ther. 16, R110 (2014).
Goldring, M. B., Tsuchimochi, K. & Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 97, 33–44 (2006).
Blanco, F. J. & Ruiz-Romero, C. New targets for disease modifying osteoarthritis drugs: chondrogenesis and Runx1. Ann. Rheum. Dis. 72, 631–634 (2013).
Georgi, N. et al. Differentiation of mesenchymal stem cells under hypoxia and normoxia: lipid profiles revealed by time-of-flight secondary ion mass spectrometry and multivariate analysis. Anal. Chem. 87, 3981–3988 (2015).
Scanzello, C. R. Pathologic and pathogenic processes in osteoarthritis: the effects of synovitis. HSS J. 8, 20–22 (2012).
Bondeson, J. et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 62, 647–657 (2010).
Cillero-Pastor, B., Eijkel, G. B., Blanco, F. J. & Heeren, R. M. Protein classification and distribution in osteoarthritic human synovial tissue by matrix-assisted laser desorption ionization mass spectrometry imaging. Anal. Bioanal. Chem. 407, 2213–2222 (2015).
Kriegsmann, M. et al. MALDI MS imaging as a powerful tool for investigating synovial tissue. Scand. J. Rheumatol. 41, 305–309 (2012).
Berntorp, E. & Shapiro, A. D. Modern haemophilia care. Lancet 379, 1447–1456 (2012).
Rodriguez-Merchan, E. C. Cartilage damage in the haemophilic joints: pathophysiology, diagnosis and management. Blood Coagul. Fibrinolysis 23, 179–183 (2012).
Roosendaal, G. & Lafeber, F. P. Blood-induced joint damage in hemophilia. Semin. Thromb. Hemost. 29, 37–42 (2003).
Roosendaal, G. & Lafeber, F. P. Pathogenesis of haemophilic arthropathy. Haemophilia 12 (Suppl. 3), 117–121 (2006).
Valentino, L. A. Blood-induced joint disease: the pathophysiology of hemophilic arthropathy. J. Thromb. Haemost. 8, 1895–1902 (2010).
Jansen, N. W. et al. The combination of the biomarkers urinary C-terminal telopeptide of type II collagen, serum cartilage oligomeric matrix protein, and serum chondroitin sulfate 846 reflects cartilage damage in hemophilic arthropathy. Arthritis Rheum. 60, 290–298 (2009).
Gerstner, G. et al. Prevalence and risk factors associated with decreased bone mineral density in patients with haemophilia. Haemophilia 15, 559–565 (2009).
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 285, 785–795 (2001).
Kriegsmann, M. et al. MALDI imaging of predictive ferritin, fibrinogen and proteases in haemophilic arthropathy. Haemophilia 20, 446–453 (2014).
Roschger, P., Misof, B., Paschalis, E., Fratzl, P. & Klaushofer, K. Changes in the degree of mineralization with osteoporosis and its treatment. Curr. Osteoporos Rep. 12, 338–350 (2014).
Yu, D. G. et al. Dynamic alterations in microarchitecture, mineralization and mechanical property of subchondral bone in rat medial meniscal tear model of osteoarthritis. Chin. Med. J. (Engl.) 128, 2879–2886 (2015).
Costa, A. M. et al. Bone mineralization in Turner syndrome: a transverse study of the determinant factors in 58 patients. J. Bone Miner. Metab. 20, 294–297 (2002).
Andrade, A. C. et al. Hormones and genes of importance in bone physiology and their influence on bone mineralization and growth in Turner syndrome. Horm. Res. Paediatr. 73, 161–165 (2010).
Fratzl-Zelman, N., Misof, B. M., Klaushofer, K. & Roschger, P. Bone mass and mineralization in osteogenesis imperfecta. Wien Med. Wochenschr. 165, 271–277 (2015).
Schaepe, K. et al. Assessment of different sample preparation routes for mass spectrometric monitoring and imaging of lipids in bone cells via ToF-SIMS. Biointerphases 10, 019016 (2015).
Henss, A., Hild, A., Rohnke, M., Wenisch, S. & Janek, J. Time of flight secondary ion mass spectrometry of bone-Impact of sample preparation and measurement conditions. Biointerphases 11, 02A302 (2015).
Henss, A. et al. Applicability of ToF-SIMS for monitoring compositional changes in bone in a long-term animal model. J. R. Soc. Interface 10, 20130332 (2013).
Kokesch-Himmelreich, J., Schumacher, M., Rohnke, M., Gelinsky, M. & Janek, J. ToF-SIMS analysis of osteoblast-like cells and their mineralized extracellular matrix on strontium enriched bone cements. Biointerphases 8, 17 (2013).
Malmberg, P., Bexell, U., Eriksson, C., Nygren, H. & Richter, K. Analysis of bone minerals by time-of-flight secondary ion mass spectrometry: a comparative study using monoatomic and cluster ions sources. Rapid Commun. Mass Spectrom. 21, 745–749 (2007).
Malmberg, P. & Nygren, H. Methods for the analysis of the composition of bone tissue, with a focus on imaging mass spectrometry (TOF-SIMS). Proteomics 8, 3755–3762 (2008).
Marie, P. J., Ammann, P., Boivin, G. & Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 69, 121–129 (2001).
Lazar, A. N. et al. Time-of-flight secondary ion mass spectrometry (TOF-SIMS) imaging reveals cholesterol overload in the cerebral cortex of Alzheimer disease patients. Acta Neuropathol. 125, 133–144 (2013).
Solé-Domènech, S. et al. Localization of cholesterol, amyloid and glia in Alzheimer's disease transgenic mouse brain tissue using time-of-flight secondary ion mass spectrometry (ToF-SIMS) and immunofluorescence imaging. Acta Neuropathol. 125, 145–157 (2013).
Schwartz, S. A. et al. Proteomic-based prognosis of brain tumor patients using direct-tissue matrix-assisted laser desorption ionization mass spectrometry. Cancer Res. 65, 7674–7681 (2005).
Schwamborn, K. et al. Identifying prostate carcinoma by MALDI-imaging. Int. J. Mol. Med. 20, 155–159 (2007).
Caprioli, R. M. Deciphering protein molecular signatures in cancer tissues to aid in diagnosis, prognosis, and therapy. Cancer Res. 65, 10642–10645 (2005).
Cornett, D. S. et al. A novel histology-directed strategy for MALDI-MS tissue profiling that improves throughput and cellular specificity in human breast cancer. Mol. Cell Proteom. 5, 1975–1983 (2006).
Frohlich, S. M. et al. Mass spectrometric imaging of in vivo protein and lipid adsorption on biodegradable vascular replacement systems. Analyst 140, 6089–6099 (2015).
Pellegatti, M. & Pagliarusco, S. Drug and metabolite concentrations in tissues in relationship to tissue adverse findings: a review. Expert Opin. Drug Metab. Toxicol. 7, 137–146 (2011).
Solon, E. G., Schweitzer, A., Stoeckli, M. & Prideaux, B. Autoradiography, MALDI-MS, and SIMS-MS imaging in pharmaceutical discovery and development. AAPS J. 12, 11–26 (2010).
Sugihara, Y. et al. A new look at drugs targeting malignant melanoma—an application for mass spectrometry imaging. Proteomics 14, 1963–1970 (2014).
Seeley, E. H. et al. Co-registration of multi-modality imaging allows for comprehensive analysis of tumor-induced bone disease. Bone 61, 208–216 (2014).
Patterson, N. H. et al. 3D imaging mass spectrometry of lipids in atherosclerotic plaques: Open-source methods for reconstruction and analysis. Proteomics 16, 1642–1651 (2016).
Cole, L. M. & Clench, M. R. Mass spectrometry imaging for the proteomic study of clinical tissue. Proteom. Clin. Appl. 9, 335–341 (2015).
Levenson, R. M., Borowsky, A. D. & Angelo, M. Immunohistochemistry and mass spectrometry for highly multiplexed cellular molecular imaging. Lab. Invest. 95, 397–405 (2015).
Giesen, C. et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods 11, 417–422 (2014).
Angelo, M. et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 20, 436–442 (2014).
Pitzalis, C., Kelly, S. & Humby, F. New learnings on the pathophysiology of RA from synovial biopsies. Curr. Opin. Rheumatol. 25, 334–344 (2013).
Astorri, E., Nerviani, A., Bombardieri, M. & Pitzalis, C. Towards a stratified targeted approach with biologic treatments in rheumatoid arthritis: role of synovial pathobiology. Curr. Pharm. Des. 21, 2216–2224 (2015).
Humby, F. et al. Use of ultrasound-guided small joint biopsy to evaluate the histopathologic response to rheumatoid arthritis therapy: recommendations for application to clinical trials. Arthritis Rheumatol. 67, 2601–2610 (2015).
Louie, K. B. & Northen, T. R. Metabolic imaging using nanostructure-initiator mass spectrometry (NIMS). Methods Mol. Biol. 1198, 313–329 (2014).
Nemes, P. & Vertes, A. Laser ablation electrospray ionization for atmospheric pressure, in vivo, and imaging mass spectrometry. Anal. Chem. 79, 8098–8106 (2007).
Nemes, P. & Vertes, A. Laser ablation electrospray ionization for atmospheric pressure molecular imaging mass spectrometry. Methods Mol. Biol. 656, 1 59–171 (2010).
Martin, N. J., Griffiths, R. L., Edwards, R. L. & Cooper, H. J. Native liquid extraction surface analysis mass spectrometry: analysis of noncovalent protein complexes directly from dried substrates. J. Am. Soc. Mass Spectrom. 26, 1320–1327 (2015).
Himmelsbach, M., Varesio, E. & Hopfgartner, G. Liquid extraction surface analysis (LESA) of hydrophobic TLC plates coupled to chip-based nanoelectrospray high-resolution mass spectrometry. Chimia (Aarau) 68, 150–154 (2014).
Acknowledgements
The authors thank Berta Cillero-Pastor, Maastricht Multimodal Molecular Imaging Institute (M4I), Maastricht, Netherlands, for kindly providing the MSI images. This work is funded by grants from Fondo Investigación Sanitaria-Spain (PI12/00329, PI14/01707, CIBER-CB06/01/0040, RETIC-RIER-RD12/0009/0018). B.R. is funded by Xunta de Galicia (IN606B-2016/004), and C.R.R. by the Miguel Servet II program from Fondo Investigación Sanitaria-Spain (CPII15/0013). The Proteomics Unit belongs to ProteoRed, PRB2-ISCIII, supported by grant PT13/0001.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, discussed its content, and wrote, reviewed, and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rocha, B., Ruiz-Romero, C. & Blanco, F. Mass spectrometry imaging: a novel technology in rheumatology. Nat Rev Rheumatol 13, 52–63 (2017). https://doi.org/10.1038/nrrheum.2016.184
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2016.184
This article is cited by
-
CircRREB1 mediates lipid metabolism related senescent phenotypes in chondrocytes through FASN post-translational modifications
Nature Communications (2023)
-
Peptidomics
Nature Reviews Methods Primers (2023)
-
PPARα−ACOT12 axis is responsible for maintaining cartilage homeostasis through modulating de novo lipogenesis
Nature Communications (2022)
-
Round robin study of formalin-fixed paraffin-embedded tissues in mass spectrometry imaging
Analytical and Bioanalytical Chemistry (2018)