Key Points
-
Autoimmunity and autoinflammatory disorders are distinct in terms of the predominance of underlying innate or adaptive immune mechanisms, therapeutic responses to biologic agents and cytokine gene expression patterns (signatures) in blood
-
When not leading to autoimmunity, autoinflammatory diseases have an IL-1β/IL-18-dominated signature, whereas autoimmune diseases are driven by type I interferon (IFN)
-
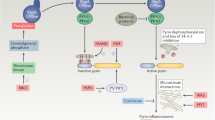
IL-1β and type I IFN counter-regulate each other by activating selective metabolic signalling pathways that interfere with adaptive immune responses
-
Hypotheses that have been proposed to explain autoimmunity initiation include molecular mimicry, for which data are limited, and conformational changes in native proteins, which can drive immunogenic self-peptide presentation
-
The current 'immunoediting' hypothesis suggests that cancer cells harbouring somatic mutations trigger adaptive immunity, including cross-reactive antibody responses
-
In addition to somatic mutations, conformational changes in proteins can result from alternative reading frames and post-translational modifications, thereby adjusting the immunogenicity of T-cell responses
Abstract
Rheumatic diseases can be divided in two groups, autoinflammatory and autoimmune disorders. The clinical presentation of both types of diseases overlap, but the pathological pathways underlying rheumatic autoinflammation and autoimmunity are distinct and are the subject of ongoing research. There are a number of ways in which these groups of diseases differ in terms of disease mechanisms and therapeutic responses. First, autoinflammatory diseases are driven by endogenous danger signals, metabolic mediators and cytokines, whereas autoimmunity involves the activation of T and B cells, the latter requiring V-(D)-J recombination of receptor-chain gene segments for maturation. Second, the efficacy of biologic agents directed against proinflammatory cytokines (for example IL-1β and TNF) also highlights differences between autoinflammatory and autoimmune processes. Finally, whereas autoinflammatory diseases are mostly driven by inflammasome-induced IL-1β and IL-18 production, autoimmune diseases are associated with type I interferon (IFN) signatures in blood. In this Review, we provide an overview of the monocyte intracellular pathways that drive autoinflammation and autoimmunity. We convey recent findings on how the type I IFN pathway can modulate IL-1β signalling (and vice versa), and discuss why IL-1β-mediated autoinflammatory diseases do not perpetuate into autoimmunity. The origins of intracellular autoantigens in autoimmune disorders are also discussed. Finally, we suggest how new mechanistic knowledge of autoinflammatory and autoimmune diseases might help improve treatment strategies to benefit patient care.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McGonagle, D. & McDermott, M. F. A proposed classification of the immunological diseases. PLoS Med. 3, e297 (2006).
Maria, A. T. et al. Adult onset Still's disease (AOSD) in the era of biologic therapies: dichotomous view for cytokine and clinical expressions. Autoimmun. Rev. 13, 1149–1159 (2014).
Correll, C. K. & Binstadt, B. A. Advances in the pathogenesis and treatment of systemic juvenile idiopathic arthritis. Pediatr. Res. 75, 176–183 (2014).
Singh, J. A. et al. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview. CMAJ 181, 787–796 (2009).
Quartier, P. et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann. Rheum. Dis. 70, 747–754 (2011).
Higgs, B. W. et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann. Rheum. Dis. 70, 2029–2036 (2011).
Lubbers, J. et al. The type I IFN signature as a biomarker of preclinical rheumatoid arthritis. Ann. Rheum. Dis. 72, 776–780 (2013).
Church, L. D., Cook, G. P. & McDermott, M. F. Primer: inflammasomes and interleukin 1β in inflammatory disorders. Nat. Clin. Pract. Rheumatol. 4, 34–42 (2008).
Richez, C. et al. TLR4 ligands induce IFN-α production by mouse conventional dendritic cells and human monocytes after IFN-β priming. J. Immunol. 182, 820–828 (2009).
Bauernfeind, F. G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Wright, H. L., Moots, R. J. & Edwards, S. W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 10, 593–601 (2014).
Lech, M. et al. NLRP3 and ASC suppress lupus-like autoimmunity by driving the immunosuppressive effects of TGF-β receptor signalling. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2014-205496.
Yang, Q. et al. Deregulated NLRP3 and NLRP1 inflammasomes and their correlations with disease activity in systemic lupus erythematosus. J. Rheumatol. 41, 444–452 (2014).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
van der Burgh, R. et al. Defects in mitochondrial clearance predispose human monocytes to interleukin-1β hypersecretion. J. Biol. Chem. 289, 5000–5012 (2014).
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013).
Kariko, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).
Lane, N. Energetics and genetics across the prokaryote-eukaryote divide. Biol. Direct 6, 35 (2011).
Pallen, M. J. Time to recognise that mitochondria are bacteria? Trends Microbiol. 19, 58–64 (2011).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Allam, R. et al. Cutting edge: cyclic polypeptide and aminoglycoside antibiotics trigger IL-1β secretion by activating the NLRP3 inflammasome. J. Immunol. 186, 2714–2718 (2011).
Iyer, S. S. et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39, 311–323 (2013).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Hornung, V., Hartmann, R., Ablasser, A. & Hopfner, K. P. OAS proteins and cGAS: unifying concepts in sensing and responding to cytosolic nucleic acids. Nat. Rev. Immunol. 14, 521–528 (2014).
Honda, K., Yanai, H., Takaoka, A. & Taniguchi, T. Regulation of the type I IFN induction: a current view. Int. Immunol. 17, 1367–1378 (2005).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011).
Radwan, M. et al. Tyrosine kinase 2 controls IL-1β production at the translational level. J. Immunol. 185, 3544–3553 (2010).
Mayer-Barber, K. D. et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 511, 99–103 (2014).
Chiu, Y. H., Macmillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Mitoma, H. et al. The DHX33 RNA helicase senses cytosolic RNA and activates the NLRP3 inflammasome. Immunity 39, 123–135 (2013).
Kowalinski, E. et al. Structural basis for the activation of innate immune pattern-recognition receptor RIG-I by viral RNA. Cell 147, 423–435 (2011).
Peisley, A., Wu, B., Xu, H., Chen, Z. J. & Hur, S. Structural basis for ubiquitin-mediated antiviral signal activation by RIG.-I. Nature 509, 110–114 (2014).
Gack, M. U. et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446, 916–920 (2007).
Tschopp, J. & Schroder, K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 (2010).
Py, B. F., Kim, M. S., Vakifahmetoglu-Norberg, H. & Yuan, J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol. Cell 49, 331–338 (2013).
Juliana, C. et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 287, 36617–36622 (2012).
Cai, X. et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell 156, 1207–1222 (2014).
Subramanian, N., Natarajan, K., Clatworthy, M. R., Wang, Z. & Germain, R. N. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell 153, 348–361 (2013).
Hou, F. et al. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 (2011).
Park, S. et al. The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity. J. Immunol. 191, 4358–4366 (2013).
Rodgers, M. A. et al. The linear ubiquitin assembly complex (LUBAC) is essential for NLRP3 inflammasome activation. J. Exp. Med. 211, 1333–1347 (2014).
Liu, S. et al. MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades. eLife 2, e00785 (2013).
Wynne, C. et al. TRIM68 negatively regulates IFN-β production by degrading TRK fused gene, a novel driver of IFN-β downstream of anti-viral detection systems. PLoS ONE 9, e101503 (2014).
Shao W, H. B., Shu, D. H., Priest, S. O. & Cohen, P. L. Aggregation of MAVS antiviral protein suggests a mechanism for increased type I interferon production in SLE. Arthritis Res. Ther. 16 (Suppl. 1), 23 (2014).
Sanz, J. M. & Di Virgilio, F. Kinetics and mechanism of ATP-dependent IL-1β release from microglial cells. J. Immunol. 164, 4893–4898 (2000).
Ouyang, X. et al. Adenosine is required for sustained inflammasome activation via the A2A receptor and the HIF-1α pathway. Nat. Commun. 4, 2909 (2013).
Wynosky-Dolfi, M. A. et al. Oxidative metabolism enables Salmonella evasion of the NLRP3 inflammasome. J. Exp. Med. 211, 653–668 (2014).
Rodriguez-Prados, J. C. et al. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 185, 605–614 (2010).
Pearce, E. L. & Pearce, E. J. Metabolic pathways in immune cell activation and quiescence. Immunity 38, 633–643 (2013).
Ruiz-Garcia, A. et al. Cooperation of adenosine with macrophage Toll-4 receptor agonists leads to increased glycolytic flux through the enhanced expression of PFKFB3 gene. J. Biol. Chem. 286, 19247–19258 (2011).
Kim, S. et al. Global metabolite profiling of synovial fluid for the specific diagnosis of rheumatoid arthritis from other inflammatory arthritis. PLoS ONE 9, e97501 (2014).
Gao, W. et al. Hypoxia and STAT3 signalling interactions regulate pro-inflammatory pathways in rheumatoid arthritis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2013-204105.
Hollander, A. P., Corke, K. P., Freemont, A. J. & Lewis, C. E. Expression of hypoxia-inducible factor 1α by macrophages in the rheumatoid synovium: implications for targeting of therapeutic genes to the inflamed joint. Arthritis Rheum. 44, 1540–1544 (2001).
Koivunen, P. et al. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J. Biol. Chem. 282, 4524–4532 (2007).
Boyle, D. L. et al. The JAK inhibitor tofacitinib suppresses synovial JAK1–STAT signalling in rheumatoid arthritis. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2014-20602.
Pontillo, A., Paoluzzi, E. & Crovella, S. The inhibition of mevalonate pathway induces upregulation of NALP3 expression: new insight in the pathogenesis of mevalonate kinase deficiency. Eur. J. Hum. Genet. 18, 844–847 (2010).
Dennis, E. A. et al. A mouse macrophage lipidome. J. Biol. Chem. 285, 39976–39985 (2010).
Cotter, D. G., Schugar, R. C. & Crawford, P. A. Ketone body metabolism and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 304, H1060–H1076 (2013).
Spann, N. J. et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151, 138–152 (2012).
Reboldi, A. et al. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 345, 679–684 (2014).
Cyster, J. G., Dang, E. V., Reboldi, A. & Yi, T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol. 14, 731–743 (2014).
Briggs, T. A. et al. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat. Genet. 43, 127–131 (2011).
Cuadrado, E. et al. Aicardi-Goutieres syndrome harbours abundant systemic and brain-reactive autoantibodies. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2014-205396.
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 371, 507–518 (2014).
Sokolovska, A. et al. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat. Immunol. 14, 543–553 (2013).
van Deventer, H. W. et al. The inflammasome component NLRP3 impairs antitumor vaccine by enhancing the accumulation of tumor-associated myeloid-derived suppressor cells. Cancer Res. 70, 10161–10169 (2010).
Neumann, S. et al. Activation of the NLRP3 inflammasome is not a feature of all particulate vaccine adjuvants. Immunol. Cell Biol. 92, 535–542 (2014).
Chen, M., Wang, H., Chen, W. & Meng, G. Regulation of adaptive immunity by the NLRP3 inflammasome. Int. Immunopharmacol. 11, 549–554 (2011).
Fuertes, M. B., Woo, S. R., Burnett, B., Fu, Y. X. & Gajewski, T. F. Type I interferon response and innate immune sensing of cancer. Trends Immunol. 34, 67–73 (2013).
Cusick, M. F., Libbey, J. E. & Fujinami, R. S. Molecular mimicry as a mechanism of autoimmune disease. Clin. Rev. Allergy Immunol. 42, 102–111 (2012).
Bach, J. F. Infections and autoimmune diseases. J. Autoimmun. 25 (Suppl.), 74–80 (2005).
Joseph, C. G. et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 343, 152–157 (2014).
Kreier, J. Infection, resistance, and immunity, 2nd Edition (Taylor & Francis, 2001).
de Graaf, K. L., Albert, M. & Weissert, R. Autoantigen conformation influences both B- and T-cell responses and encephalitogenicity. J. Biol. Chem. 287, 17206–17213 (2012).
de Laat, B. et al. Immune responses against domain I of β2-glycoprotein I are driven by conformational changes: domain I of β2-glycoprotein I harbors a cryptic immunogenic epitope. Arthritis Rheum. 63, 3960–3968 (2011).
Plotz, P. H. The autoantibody repertoire: searching for order. Nat. Rev. Immunol. 3, 73–78 (2003).
Backes, C. et al. Immunogenicity of autoantigens. BMC Genomics 12, 340 (2011).
Hayter, S. M. & Cook, M. C. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun. Rev. 11, 754–765 (2012).
Stadler, M. B., Arnold, D., Frieden, S., Luginbuhl, S. & Stadler, B. M. Single nucleotide polymorphisms as a prerequisite for autoantigens. Eur. J. Immunol. 35, 371–378 (2005).
Yamanishi, Y. et al. p53 tumor suppressor gene mutations in fibroblast-like synoviocytes from erosion synovium and non-erosion synovium in rheumatoid arthritis. Arthritis Res. Ther. 7, R12–R18 (2005).
Tak, P. P. et al. p53 overexpression in synovial tissue from patients with early and longstanding rheumatoid arthritis compared with patients with reactive arthritis and osteoarthritis. Arthritis Rheum. 42, 948–953 (1999).
Lee, S. H. et al. Microsatellite instability and suppressed DNA repair enzyme expression in rheumatoid arthritis. J. Immunol. 170, 2214–2220 (2003).
Singh, N. et al. Genomic alterations in abnormal neutrophils isolated from adult patients with systemic lupus erythematosus. Arthritis Res. Ther. 16, R165 (2014).
Ekstrom, K. et al. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum. 48, 963–970 (2003).
Bernatsky, S., Ramsey-Goldman, R. & Clarke, A. Malignancy and autoimmunity. Curr. Opin. Rheumatol. 18, 129–134 (2006).
Mariette, X. et al. Anti-p53 antibodies are rarely detected in serum of patients with rheumatoid arthritis and Sjogren's syndrome. J. Rheumatol. 26, 1672–1675 (1999).
Chauhan, R., Handa, R., Das, T. P. & Pati, U. Over-expression of TATA binding protein (TBP) and p53 and autoantibodies to these antigens are features of systemic sclerosis, systemic lupus erythematosus and overlap syndromes. Clin. Exp. Immunol. 136, 574–584 (2004).
Hansen, J. E. et al. Targeting cancer with a lupus autoantibody. Sci. Transl. Med. 4, 157ra142 (2012).
Yewdell, J. W., Anton, L. C. & Bennink, J. R. Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules? J. Immunol. 157, 1823–1826 (1996).
Rock, K. L., Farfan-Arribas, D. J., Colbert, J. D. & Goldberg, A. L. Re-examining class-I presentation and the DRiP hypothesis. Trends Immunol. 35, 144–152 (2014).
Michel, A. M. et al. Observation of dually decoded regions of the human genome using ribosome profiling data. Genome Res. 22, 2219–2229 (2012).
Saulquin, X. et al. +1 Frameshifting as a novel mechanism to generate a cryptic cytotoxic T lymphocyte epitope derived from human interleukin 10. J. Exp. Med. 195, 353–358 (2002).
Zook, M. B., Howard, M. T., Sinnathamby, G., Atkins, J. F. & Eisenlohr, L. C. Epitopes derived by incidental translational frameshifting give rise to a protective CTL response. J. Immunol. 176, 6928–6934 (2006).
Berger, C. T. et al. Viral adaptation to immune selection pressure by HLA class I-restricted CTL responses targeting epitopes in HIV frameshift sequences. J. Exp. Med. 207, 61–75 (2010).
Koenig, P. A. & Ploegh, H. L. Protein quality control in the endoplasmic reticulum. F1000Prime Rep. 6, 49 (2014).
Feige, M. J. & Hendershot, L. M. Quality control of integral membrane proteins by assembly-dependent membrane integration. Mol. Cell 51, 297–309 (2013).
Huang, L., Kuhls, M. C. & Eisenlohr, L. C. Hydrophobicity as a driver of MHC class I antigen processing. EMBO J. 30, 1634–1644 (2011).
Bergès, J., Trouillas, P. & Houée-Levin, C. Oxidation of protein tyrosine or methionine residues: From the amino acid to the peptide. J. Phys. Conf. Ser. 261, 8 (2011).
Hsu, H. T. et al. Endoplasmic reticulum targeting alters regulation of expression and antigen presentation of proinsulin. J. Immunol. 192, 4957–4966 (2014).
Granados, D. P. et al. ER stress affects processing of MHC class I-associated peptides. BMC Immunol. 10, 10 (2009).
Herzog, J., Maekawa, Y., Cirrito, T. P., Illian, B. S. & Unanue, E. R. Activated antigen-presenting cells select and present chemically modified peptides recognized by unique CD4 T cells. Proc. Natl Acad. Sci. USA 102, 7928–7933 (2005).
Wang, G., Pierangeli, S. S., Papalardo, E., Ansari, G. A. & Khan, M. F. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: correlation with disease activity. Arthritis Rheum. 62, 2064–2072 (2010).
Martinon, F. & Aksentijevich, I. New players driving inflammation in monogenic autoinflammatory diseases. Nat. Rev. Rheumatol. 11, 11–20 (2015).
Author information
Authors and Affiliations
Contributions
T.S.v.K researched data for the article. T.S.v.K., T.R.D.J.R and M.B. wrote the manuscript. All authors made a substantial contribution to the discussion of the review and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
van Kempen, T., Wenink, M., Leijten, E. et al. Perception of self: distinguishing autoimmunity from autoinflammation. Nat Rev Rheumatol 11, 483–492 (2015). https://doi.org/10.1038/nrrheum.2015.60
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.60
This article is cited by
-
Unveiling the dark side of glucose-regulated protein 78 (GRP78) in cancers and other human pathology: a systematic review
Molecular Medicine (2023)
-
Regulation of immunological tolerance by the p53-inhibitor iASPP
Cell Death & Disease (2023)
-
Paradigm Shift in Diagnosis and Targeted Therapy in Recurrent Pericarditis
Current Cardiology Reports (2023)
-
Dysregulation of miRNA-30e-3p targeting IL-1β in an international cohort of systemic autoinflammatory disease patients
Journal of Molecular Medicine (2023)
-
Rilonacept (Interleukin-1 Inhibition) for the Treatment of Pericarditis
Current Cardiology Reports (2022)