Key Points
-
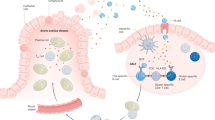
Coeliac disease and rheumatoid arthritis are multifactorial HLA-associated diseases
-
Immune responses to enzymatically modified protein antigens are a hallmark of both these diseases
-
Environmental insults are probably key to breaking of immune tolerance to enzymatically modified protein antigens
-
B-cell to T-cell presentation of target enzymatically modified protein antigens provides a powerful amplification loop sustaining the autoimmune disease process
-
This paradigm provides multiple targets for specific interventions aimed at reinstating immune tolerance to enzymatically modified protein antigens
Abstract
Rheumatoid arthritis (RA) and coeliac disease are inflammatory diseases that both have a strong association with class II HLAs: individuals carrying HLA-DQ2.5 and/or HLA-DQ8 alleles have an increased risk of developing coeliac disease, whereas those carrying HLA-DR shared epitope alleles exhibit an increased risk of developing RA. Although the molecular basis of the association with specific HLA molecules in RA remains poorly defined, an immune response against post-translationally modified protein antigens is a hallmark of each disease. In RA, understanding of the pathogenetic role of B-cell responses to citrullinated antigens, including vimentin, fibrinogen and α-enolase, is rapidly growing. Moreover, insight into the role of HLAs in the pathogenesis of coeliac disease has been considerably advanced by the identification of T-cell responses to deamidated gluten antigens presented in conjunction with predisposing HLA-DQ2.5 molecules. This article briefly reviews these advances and draws parallels between the immune mechanisms leading to RA and coeliac disease, which point to a crucial role for T-cell–B-cell cooperation in the development of full-blown disease. Finally, the ways in which these novel insights are being exploited therapeutically to re-establish tolerance in patients with RA and coeliac disease are described.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thorsby, E. Invited anniversary review: HLA associated diseases. Hum. Immunol. 53, 1–11 (1997).
Stastny, P. Association of the B-cell alloantigen DRW4 with rheumatoid arthritis. N. Engl. J. Med. 298, 869–871 (1978).
Gregersen, P. K., Silver, J. & Winchester, R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30, 1205–1213 (1987).
Raychaudhuri, S. et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat. Genet. 44, 291–296 (2012).
Chicz, R. M. et al. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J. Exp. Med. 178, 27–47 (1993).
Kirschmann, D. A. et al. Naturally processed peptides from rheumatoid arthritis associated and non-associated HLA-DR alleles. J. Immunol. 155, 5655–5662 (1995).
Scally, S. W. et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J. Exp. Med. 210, 2569–2582 (2013).
Huizinga, T. W. et al. Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis Rheum. 52, 3433–3438 (2005).
van Venrooij, W. J., van Beers, J. J. & Pruijn, G. J. Anti-CCP antibodies: the past, the present and the future. Nat. Rev. Rheumatol. 7, 391–398 (2011).
Suzuki, A. et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat. Genet. 34, 395–402 (2003).
Plenge, R. M. et al. Replication of putative candidate-gene associations with rheumatoid arthritis in >4,000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4. Am. J. Hum. Genet. 77, 1044–1060 (2005).
van der Woude, D. et al. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Ann. Rheum. Dis. 69, 1554–1561 (2010).
He, X. et al. T cell receptors recognizing type II collagen in HLA-DR-transgenic mice characterized by highly restricted V β usage. Arthritis Rheum. 50, 1996–2004 (2004).
Fritsch, R. et al. Characterization of autoreactive T cells to the autoantigens heterogeneous nuclear ribonucleoprotein A2 (RA33) and filaggrin in patients with rheumatoid arthritis. J. Immunol. 169, 1068–1076 (2002).
Blass, S. et al. The stress protein BiP is overexpressed and is a major B and T cell target in rheumatoid arthritis. Arthritis Rheum. 44, 761–771 (2001).
Verpoort, K. N. et al. Association of HLA-DR3 with anti-cyclic citrullinated peptide antibody-negative rheumatoid arthritis. Arthritis Rheum. 52, 3058–3062 (2005).
Irigoyen, P. et al. Regulation of anti-cyclic citrullinated peptide antibodies in rheumatoid arthritis: contrasting effects of HLA-DR3 and the shared epitope alleles. Arthritis Rheum. 52, 3813–3818 (2005).
Oka, S. et al. Protective effect of the HLA-DRB1*13:02 allele in Japanese rheumatoid arthritis patients. PLoS ONE 9, e99453 (2014).
van der Woude, D. et al. Protection against anti-citrullinated protein antibody-positive rheumatoid arthritis is predominantly associated with HLA-DRB1*1301: a meta-analysis of HLA-DRB1 associations with anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis in four European populations. Arthritis Rheum. 62, 1236–1245 (2010).
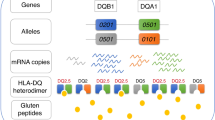
Tjon, J. M., van Bergen, J. & Koning, F. Celiac disease: how complicated can it get? Immunogenetics 62, 641–651 (2010).
Abadie, V., Sollid, L. M., Barreiro, L. B. & Jabri, B. Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annu. Rev. Immunol. 29, 493–525 (2011).
Vader, W. et al. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc. Natl Acad. Sci. USA 100, 12390–12395 (2003).
Kumar, V., Wijmenga, C. & Withoff, S. From genome-wide association studies to disease mechanisms: celiac disease as a model for autoimmune diseases. Semin. Immunopathol. 34, 567–580 (2012).
Sollid, L. M., Qiao, S. W., Anderson, R. P., Gianfrani, C. & Koning, F. Nomenclature and listing of celiac disease relevant gluten T-cell epitopes restricted by HLA-DQ molecules. Immunogenetics 64, 455–460 (2012).
Anderson, R. P. et al. T cells in peripheral blood after gluten challenge in coeliac disease. Gut 54, 1217–1223 (2005).
Tye-Din, J. A. et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci. Transl. Med. 2, 41ra51 (2010).
Fina, D. et al. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut 57, 887–892 (2008).
Bodd, M. et al. HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol. 3, 594–601 (2010).
van Leeuwen, M. A. et al. Increased production of interleukin-21, but not interleukin-17A, in the small intestine characterizes pediatric celiac disease. Mucosal Immunol. 6, 1202–1213 (2013).
Shan, L. et al. Structural basis for gluten intolerance in celiac sprue. Science 297, 2275–2279 (2002).
Vader, L. W. et al. Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J. Exp. Med. 195, 643–649 (2002).
van de Wal, Y. et al. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 161, 1585–1588 (1998).
Molberg, O. et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 4, 713–717 (1998).
Henderson, K. N. et al. A structural and immunological basis for the role of human leukocyte antigen DQ8 in celiac disease. Immunity 27, 23–34 (2007).
Petersen, J., Purcell, A. W. & Rossjohn, J. Post-translationally modified T cell epitopes: immune recognition and immunotherapy. J. Mol. Med. (Berl.) 87, 1045–1051 (2009).
Kim, C. Y., Quarsten, H., Bergseng, E., Khosla, C. & Sollid, L. M. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease. Proc. Natl Acad. Sci. USA 101, 4175–4179 (2004).
Fallang, L. E. et al. Differences in the risk of celiac disease associated with HLA-DQ2.5 or HLA-DQ2.2 are related to sustained gluten antigen presentation. Nat. Immunol. 10, 1096–1101 (2009).
Vader, W. et al. The gluten response in children with celiac disease is directed toward multiple gliadin and glutenin peptides. Gastroenterology 122, 1729–1737 (2002).
Arentz-Hansen, H. et al. The intestinal T cell response to α-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J. Exp. Med. 191, 603–612 (2000).
Anderson, R. P., Degano, P., Godkin, A. J., Jewell, D. P. & Hill, A. V. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat. Med. 6, 337–342 (2000).
Vader, L. W. et al. Characterization of cereal toxicity for celiac disease patients based on protein homology in grains. Gastroenterology 125, 1105–1113 (2003).
Turner, S. J., Doherty, P. C., McCluskey, J. & Rossjohn, J. Structural determinants of T-cell receptor bias in immunity. Nat. Rev. Immunol. 6, 883–894 (2006).
Petersen, J. et al. T-cell receptor recognition of HLA-DQ2–gliadin complexes associated with celiac disease. Nat. Struct. Mol. Biol. 21, 480–488 (2014).
Broughton, S. E. et al. Biased T cell receptor usage directed against human leukocyte antigen DQ8-restricted gliadin peptides is associated with celiac disease. Immunity 37, 611–621 (2012).
Qiao, S. W., Christophersen, A., Lundin, K. E. & Sollid, L. M. Biased usage and preferred pairing of α- and β-chains of TCRs specific for an immunodominant gluten epitope in coeliac disease. Int. Immunol. 26, 13–19 (2014).
Qiao, S. W. et al. Posttranslational modification of gluten shapes TCR usage in celiac disease. J. Immunol. 187, 3064–3071 (2011).
Gras, S. et al. A structural voyage toward an understanding of the MHC-I-restricted immune response: lessons learned and much to be learned. Immunol. Rev. 250, 61–81 (2012).
Rossjohn, J. et al. T cell antigen receptor recognition of antigen-presenting molecules. Annu. Rev. Immunol. http://dx.doi.org/10.1146/annurev-immunol-032414-112334.
Yin, Y., Li, Y. & Mariuzza, R. A. Structural basis for self-recognition by autoimmune T-cell receptors. Immunol. Rev. 250, 32–48 (2012).
Quarsten, H. et al. Staining of celiac disease-relevant T cells by peptide–DQ2 multimers. J. Immunol. 167, 4861–4868 (2001).
Bodd, M. et al. Direct cloning and tetramer staining to measure the frequency of intestinal gluten-reactive T cells in celiac disease. Eur. J. Immunol. 43, 2605–2612 (2013).
Law, S. C., Benham, H., Reid, H. H., Rossjohn, J. & Thomas, R. Identification of self-antigen-specific T cells reflecting loss of tolerance in autoimmune disease underpins preventative immunotherapeutic strategies in rheumatoid arthritis. Rheum. Dis. Clin. North Am. 40, 735–752 (2014).
Verpoort, K. N. et al. Isotype distribution of anti-cyclic citrullinated peptide antibodies in undifferentiated arthritis and rheumatoid arthritis reflects an ongoing immune response. Arthritis Rheum. 54, 3799–3808 (2006).
Amara, K. et al. Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J. Exp. Med. 210, 445–455 (2013).
Verpoort, K. N. et al. Fine specificity of the anti-citrullinated protein antibody response is influenced by the shared epitope alleles. Arthritis Rheum. 56, 3949–3952 (2007).
van der Helm-van Mil, A. H. et al. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 54, 1117–1121 (2006).
McDevitt, H. The discovery of linkage between the MHC and genetic control of the immune response. Immunol. Rev. 185, 78–85 (2002).
Feitsma, A. L. et al. Identification of citrullinated vimentin peptides as T cell epitopes in HLA-DR4-positive patients with rheumatoid arthritis. Arthritis Rheum. 62, 117–125 (2010).
James, E. et al. Citrulline specific TH1 cells are increased in rheumatoid arthritis and their frequency is influenced by disease duration and therapy. Arthritis Rheumatol. 66, 1712–1722 (2014).
Law, S. C. et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res. Ther. 14, R118 (2012).
Stadinski, B. D. et al. Diabetogenic T cells recognize insulin bound to IAg7 in an unexpected, weakly binding register. Proc. Natl Acad. Sci. USA 107, 10978–10983 (2010).
Liu, G. Y. et al. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity 3, 407–415 (1995).
Salmond, R. J., Brownlie, R. J., Morrison, V. L. & Zamoyska, R. The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat. Immunol. 15, 875–883 (2014).
Maine, C. J., Marquardt, K., Cheung, J. & Sherman, L. A. PTPN22 controls the germinal center by influencing the numbers and activity of T follicular helper cells. J. Immunol. 192, 1415–1424 (2014).
Deshpande, P. et al. IL-7- and IL-15-mediated TCR sensitization enables T cell responses to self-antigens. J. Immunol. 190, 1416–1423 (2013).
Han, B. et al. Fine mapping seronegative and seropositive rheumatoid arthritis to shared and distinct HLA alleles by adjusting for the effects of heterogeneity. Am. J. Hum. Genet. 94, 522–532 (2014).
Klarenbeek, P. L. et al. Inflamed target tissue provides a specific niche for highly expanded T-cell clones in early human autoimmune disease. Ann. Rheum. Dis. 71, 1088–1093 (2012).
Ioan-Facsinay, A. et al. Anti-cyclic citrullinated peptide antibodies are a collection of anti-citrullinated protein antibodies and contain overlapping and non-overlapping reactivities. Ann. Rheum. Dis. 70, 188–193 (2011).
van de Stadt, L. A. et al. Monoclonal anti-citrullinated protein antibodies selected on citrullinated fibrinogen have distinct targets with different cross-reactivity patterns. Rheumatology (Oxford) 52, 631–635 (2013).
van Bilsen, J. H. et al. Functional regulatory immune responses against human cartilage glycoprotein-39 in health vs. proinflammatory responses in rheumatoid arthritis. Proc. Natl Acad. Sci. USA 101, 17180–17185 (2004).
Snir, O. et al. Multifunctional T cell reactivity with native and glycosylated type II collagen in rheumatoid arthritis. Arthritis Rheum. 64, 2482–2488 (2012).
Corrigall, V. M., Bodman-Smith, M. D., Brunst, M., Cornell, H. & Panayi, G. S. Inhibition of antigen-presenting cell function and stimulation of human peripheral blood mononuclear cells to express an antiinflammatory cytokine profile by the stress protein BiP: relevance to the treatment of inflammatory arthritis. Arthritis Rheum. 50, 1164–1171 (2004).
Zou, J. et al. Predominant cellular immune response to the cartilage autoantigenic G1 aggrecan in ankylosing spondylitis and rheumatoid arthritis. Rheumatology (Oxford) 42, 846–855 (2003).
Nielen, M. M. et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 50, 380–386 (2004).
Linn-Rasker, S. P. et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann. Rheum. Dis. 65, 366–371 (2006).
Klareskog, L. et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 54, 38–46 (2006).
Ioan-Facsinay, A. et al. Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum. 58, 3000–3008 (2008).
van de Stadt, L. A. et al. Development of the anti-citrullinated protein antibody repertoire prior to the onset of rheumatoid arthritis. Arthritis Rheum. 63, 3226–3233 (2011).
van de Stadt, L. A. et al. The extent of the anti-citrullinated protein antibody repertoire is associated with arthritis development in patients with seropositive arthralgia. Ann. Rheum. Dis. 70, 128–133 (2011).
Hensvold, A. H. et al. Environmental and genetic factors in the development of anticitrullinated protein antibodies (ACPAs) and ACPA-positive rheumatoid arthritis: an epidemiological investigation in twins. Ann. Rheum. Dis. 74, 375–380 (2013).
Terao, C. et al. Effects of smoking and shared epitope on the production of anti-citrullinated peptide antibody in a Japanese adult population. Arthritis Care Res. (Hoboken) 66, 1818–1827 (2014).
Dieterich, W. et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat. Med. 3, 797–801 (1997).
Osman, A. A. et al. B cell epitopes of gliadin. Clin. Exp. Immunol. 121, 248–254 (2000).
Prause, C. et al. Antibodies against deamidated gliadin as new and accurate biomarkers of childhood coeliac disease. J. Pediatr. Gastroenterol. Nutr. 49, 52–58 (2009).
Di Niro, R. et al. High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat. Med. 18, 441–445 (2012).
Steinsbø, Ø. et al. Restricted VH/VL usage and limited mutations in gluten-specific IgA of coeliac disease lesion plasma cells. Nat. Commun. 5, 4041 (2014).
Matysiak-Budnik, T. et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J. Exp. Med. 205, 143–154 (2008).
Sardy, M., Karpati, S., Merkl, B., Paulsson, M. & Smyth, N. Epidermal transglutaminase (TGase 3) is the autoantigen of dermatitis herpetiformis. J. Exp. Med. 195, 747–757 (2002).
Edwards, J. C. et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 350, 2572–2581 (2004).
Conrad, K., Roggenbuck, D., Reinhold, D. & Dorner, T. Profiling of rheumatoid arthritis associated autoantibodies. Autoimmun. Rev. 9, 431–435 (2010).
Shi, J. et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl Acad. Sci. USA 108, 17372–17377 (2011).
Willemze, A., Trouw, L. A., Toes, R. E. & Huizinga, T. W. The influence of ACPA status and characteristics on the course of RA. Nat. Rev. Rheumatol. 8, 144–152 (2012).
Laurent, L. et al. IgM rheumatoid factor amplifies the inflammatory response of macrophages induced by the rheumatoid arthritis-specific immune complexes containing anticitrullinated protein antibodies. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2013-204543.
Laurent, L. et al. Fcγ receptor profile of monocytes and macrophages from rheumatoid arthritis patients and their response to immune complexes formed with autoantibodies to citrullinated proteins. Ann. Rheum. Dis. 70, 1052–1059 (2011).
Trouw, L. A. et al. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum. 60, 1923–1931 (2009).
Harre, U. et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 122, 1791–1802 (2012).
Uysal, H. et al. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J. Exp. Med. 206, 449–462 (2009).
Kuhn, K. A. et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J. Clin. Invest. 116, 961–973 (2006).
Khandpur, R. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5, 178ra40 (2013).
Landmann, T., Kehl, G. & Bergner, R. The continuous measurement of anti-CCP-antibodies does not help to evaluate the disease activity in anti-CCP-antibody-positive patients with rheumatoid arthritis. Clin. Rheumatol. 29, 1449–1453 (2010).
Chang, C. The pathogenesis of neonatal autoimmune and autoinflammatory diseases: a comprehensive review. J. Autoimmun. 41, 100–110 (2013).
Abdullah, S. N., Farmer, E. A., Spargo, L., Logan, R. & Gully, N. Porphyromonas gingivalis peptidylarginine deiminase substrate specificity. Anaerobe 23, 102–108 (2013).
Kerkman, P. F. et al. Circulating plasmablasts/plasmacells as a source of anticitrullinated protein antibodies in patients with rheumatoid arthritis. Ann. Rheum. Dis. 72, 1259–1263 (2013).
Nissinen, R. et al. Peptidylarginine deiminase, the arginine to citrulline converting enzyme, is frequently recognized by sera of patients with rheumatoid arthritis, systemic lupus erythematosus and primary Sjögren syndrome. Scand. J. Rheumatol. 32, 337–342 (2003).
Halvorsen, E.H. et al. Serum IgG antibodies to peptidylarginine deiminase 4 in rheumatoid arthritis and associations with disease severity. Ann Rheum Dis. 67, 414–417 (2008).
Darrah, E. et al. Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Sci. Transl. Med. 5, 186ra65 (2013).
Ciccocioppo, R. et al. Gliadin and tissue transglutaminase complexes in normal and coeliac duodenal mucosa. Clin. Exp. Immunol. 134, 516–524 (2003).
Palazzo, C., Nicaise-Roland, P. & Palazzo, E. Rituximab: an effective treatment for rheumatologic and digestive symptoms of celiac disease? Joint Bone Spine 79, 422–423 (2012).
Nikiphorou, E. & Hall, F. C. First report of improvement of coeliac disease in a patient with Sjögren's syndrome treated with rituximab. Rheumatology (Oxford) 53, 1906–1907 (2014).
Thurlings, R. M. et al. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann. Rheum. Dis. 67, 917–925 (2008).
Willis, V. C. et al. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J. Immunol. 186, 4396–4404 (2011).
Michels, A. W. et al. Structure-based selection of small molecules to alter allele-specific MHC class II antigen presentation. J. Immunol. 187, 5921–5930 (2011).
Thomas, R. Dendritic cells as targets or therapeutics in rheumatic autoimmune disease. Curr. Opin. Rheumatol. 26, 211–218 (2014).
Giannoukakis, N., Phillips, B., Finegold, D., Harnaha, J. & Trucco, M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care 34, 2026–2032 (2011).
Roep, B. O. et al. Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci. Transl. Med. 5, 191ra82 (2013).
Wherrett, D. K. et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 378, 319–327 (2011).
Christophersen, A. et al. Tetramer-visualized gluten-specific CD4+ T cells in blood as a potential diagnostic marker for coeliac disease without oral gluten challenge. United European Gastroenterol. J. 2, 268–278 (2014).
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Amir el-A. D. et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol. 31, 545–552 (2013).
Su, L. F. et al. The promised land of human immunology. Cold Spring Harb. Symp. Quant. Biol. 78, 203–213 (2013).
Acknowledgements
The authors' research work is supported by the Australian Research Council, the National Health and Medical Research Council (NHMRC) of Australia and the Dutch Arthritis foundation. R.T. is supported by an NHMRC Research fellowship and Arthritis Queensland, J.R. by an NHMRC Australia Fellowship, and R.E.T. by a Vici fellowship from Nederlandse Organizatie voor Wetenschappelijk Onderzoek (NWO). The authors thank Jan Petersen for help with the drafting of the original figures.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the manuscript: researching data for the article, discussions of its content, writing and review or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.T. has filed patent applications (PCT/AU2007/001555: Compositions and methods for modulating immune responses, USA; PCT/AU2013/000303: Citrullinated aggrecan peptides for immunotherapy in rheumatoid arthritis, USA) related to technology for targeting dendritic cells to achieve antigen-specific tolerance, and is a director of Dendright, a spin-off company developing commercial vaccines that target dendritic cells to suppress autoimmune diseases. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Koning, F., Thomas, R., Rossjohn, J. et al. Coeliac disease and rheumatoid arthritis: similar mechanisms, different antigens. Nat Rev Rheumatol 11, 450–461 (2015). https://doi.org/10.1038/nrrheum.2015.59
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2015.59
This article is cited by
-
Going Against the Grains: Gluten-Free Diets in Patients Without Celiac Disease—Worthwhile or Not?
Digestive Diseases and Sciences (2019)
-
HLA and kidney disease: from associations to mechanisms
Nature Reviews Nephrology (2018)
-
Drugs and drug-like molecules can modulate the function of mucosal-associated invariant T cells
Nature Immunology (2017)
-
A biased view toward celiac disease
Mucosal Immunology (2016)
-
Where, How, and When: Positioning Posttranslational Modification Within Type 1 Diabetes Pathogenesis
Current Diabetes Reports (2016)