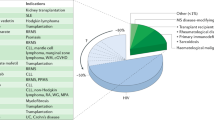
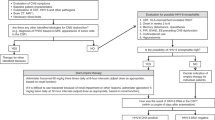
Abstract
Progressive multifocal leukoencephalopathy (PML) is a rare, opportunistic infection of the central nervous system, caused by reactivation of the ubiquitous JC virus. PML is a devastating disease that is frequently fatal, and although survival rates have improved, patients who survive PML often experience considerable neurological deficits. PML was associated with a variety of immunosuppressive therapies in the past decade, but attribution of causality is difficult owing to the presence of confounding factors and to an inadequate understanding of the underlying pathogenesis of this disease. This uncertainty has hindered efforts for shared decision-making between physicians and their patients and, in some cases, discouraged the use of potentially beneficial therapies. We propose a categorization of immunosuppressive agents according to their risk of PML to support a better-informed decision-making process when evaluating the risks and benefits of these therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Berger, J. R. et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology 80, 1430–1438 (2013).
Berger, J. R. & Houff, S. Progressive multifocal leukoencephalopathy: lessons from AIDS and natalizumab. Neurol. Res. 28, 299–305 (2006).
Kleinschmidt-DeMasters, B. K. & Tyler, K. L. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon β-1a for multiple sclerosis. N. Engl. J. Med. 353, 369–374 (2005).
Langer-Gould, A., Atlas, S. W., Green, A. J., Bollen, A. W. & Pelletier, D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353, 375–381 (2005).
Van Assche, G. et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N. Engl. J. Med. 353, 362–368 (2005).
Calabrese, L. H., Molloy, E. S., Huang, D. & Ransohoff, R. M. Progressive multifocal leukoencephalopathy in rheumatic diseases: evolving clinical and pathologic patterns of disease. Arthritis Rheum. 56, 2116–2128 (2007).
Molloy, E. S. & Calabrese, L. H. Progressive multifocal leukoencephalopathy in patients with rheumatic diseases: are patients with systemic lupus erythematosus at particular risk? Autoimmun. Rev. 8, 144–146 (2008).
Berger, J. R. The basis for modeling progressive multifocal leukoencephalopathy pathogenesis. Curr. Opin. Neurol. 24, 262–267 (2011).
Molloy, E. S. & Calabrese, L. H. Progressive multifocal leukoencephalopathy: a national estimate of frequency in systemic lupus erythematosus and other rheumatic diseases. Arthritis Rheum. 60, 3761–3765 (2009).
Calabrese, L. H. & Molloy, E. S. Progressive multifocal leucoencephalopathy in the rheumatic diseases: assessing the risks of biological immunosuppressive therapies. Ann. Rheum. Dis. 67 (Suppl. 3), iii64–iii65 (2008).
Molloy, E. S. & Calabrese, L. H. Therapy: Targeted but not trouble-free: efalizumab and PML. Nat. Rev. Rheumatol. 5, 418–419 (2009).
US Department of Health & Human Services. FDA Drug Safety and Availability [online], (2010).
US Department of Health & Human Services. FDA Drug Safety and Availability [online], (2006).
US Department of Health & Human Services. FDA Drug Safety and Availability [online], (2008).
Neff, R. T. et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation 86, 1474–1478 (2008).
Clifford, D. B. et al. Rituximab-associated progressive multifocal leukoencephalopathy in rheumatoid arthritis. Arch. Neurol. 68, 1156–1164 (2011).
Molloy, E. S. & Calabrese, L. H. Progressive multifocal leukoencephalopathy associated with immunosuppressive therapy in rheumatic diseases: evolving role of biologic therapies. Arthritis Rheum. 64, 3043–3051 (2012).
Fredericks, C., Kvam, K., Bear, J., Crabtree, G. & Josephson, S. A case of progressive multifocal leukoencephalopathy in a lupus patient treated with belimumab. Lupus 23, 711–713 (2014).
Padgett, B. L., Walker, D. L., ZuRhein, G. M., Eckroade, R. J. & Dessel, B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1, 1257–1260 (1971).
Berger, J. R. & Khalili, K. The pathogenesis of progressive multifocal leukoencephalopathy. Discov. Med. 12, 495–503 (2012).
Egli, A. et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 199, 837–46 (2009).
Gorelik, L. et al. Anti-JC virus antibodies: implications for PML risk stratification. Ann. Neurol. 68, 295–303 (2010).
Monaco, M. C., Jensen, P. N., Hou, J., Durham, L. C. & Major, E. O. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J. Virol. 72, 9918–9923 (1998).
Houff, S. & Berger, J. R. The bone marrow, B cells and JC virus. J. Neurovirol. 14, 341–343 (2008).
Houff, S. A. et al. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N. Engl. J. Med. 318, 301–305 (1988).
Chen, Y. et al. Asymptomatic reactivation of JC virus in patients treated with natalizumab. N. Engl. J. Med. 361, 1067–1074 (2009).
Marzocchetti, A. et al. Efficient in vitro expansion of JC virus-specific CD8+ T-cell responses by JCV peptide-stimulated dendritic cells from patients with progressive multifocal leukoencephalopathy. Virology 383, 173–177 (2009).
Biogen Idec. Medical Information [online], (2014).
Major, E. O. & Douek, D. C. Risk factors for rare diseases can be risky to define: PML and natalizumab. Neurology 81, 858–859 (2013).
Zaheer, F. & Berger, J. R. Treatment-related progressive multifocal leukoencephalopathy: current understanding and future steps. Ther. Adv. Drug Saf. 3, 227–239 (2012).
Vinhas de Souza, M. et al. Drug-induced PML: a global agenda for a global challenge. Clin. Pharmacol. Ther. 91, 747–750 (2012).
Molloy, E. S. PML and rheumatology: the contribution of disease and drugs. Cleve. Clin. J. Med. 78 (Suppl. 2), S28–S32 (2011).
Gheuens, S., Pierone, G., Peeters, P. & Koralnik, I. J. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J. Neurol. Neurosurg. Psychiatry 81, 247–254 (2010).
Reuben, D. B. & Tinetti, M. E. Goal-oriented patient care—an alternative health outcomes paradigm. N. Engl. J. Med. 366, 777–779 (2012).
European Commission. A guideline on summary of product characteristics [online], (2009).
Frohman, E. M. et al. JC virus in CD34+ and CD19+ cells in patients with multiple sclerosis treated with natalizumab. JAMA Neurol. 71, 596–602 (2014).
Major, E. O., Frohman, E. & Douek, D. JC viremia in natalizumab-treated patients with multiple sclerosis. N. Engl. J. Med. 368, 2240–2241 (2013).
Berger, J. R. et al. JC virus antibody status underestimates infection rates. Ann. Neurol. 74, 84–90 (2013).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching data for the article, discussions of the content, writing the article and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
L.H.C. has served as consultant to Genentech, GlaxoSmithKline and Pfizer in the area of progressive multifocal leukoencephalopathy (PML). E.M. has obtained research support from Roche, has served on advisory boards for Bristol-Myers Squibb, Pfizer and MSD, and received payment for developing educational materials relating to PML from GlaxoSmithKline. J.B. serves on the PML Adjudication Committees of Amgen, Astra Zeneca, Bristol-Myers Squibb, Eisai, Janssen, Millennium, Parexel, Pfizer, Roche and Takeda; he is a consultant to Genentech, Genzyme, Incyte, Inhibikase Therapeutics, Johnson & Johnson and Novartis. He has received grants from the PML Consortium, Biogen Idec and Novartis.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Calabrese, L., Molloy, E. & Berger, J. Sorting out the risks in progressive multifocal leukoencephalopathy. Nat Rev Rheumatol 11, 119–123 (2015). https://doi.org/10.1038/nrrheum.2014.167
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2014.167
This article is cited by
-
Evaluation of Facebook and Twitter Monitoring to Detect Safety Signals for Medical Products: An Analysis of Recent FDA Safety Alerts
Drug Safety (2017)
-
Progressive multifocal leukoencephalopathy in patients treated with fumaric acid esters: a review of 19 cases
Journal of Neurology (2017)
-
Infection and Lupus: Which Causes Which?
Current Rheumatology Reports (2016)
-
Treatment-Related Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis: A Comprehensive Review of Current Evidence and Future Needs
Drug Safety (2016)