Key Points
-
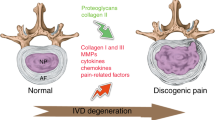
Intervertebral disc (IVD) degeneration is a common condition, affecting a large percentage of the adult population, with huge socio-economic costs
-
IVD disease is frequently associated with back, neck and radicular pain
-
Inflammatory cytokines play a major part in the pathogenesis of IVD degeneration by promoting extracellular matrix breakdown and recruitment of immune cells to the discal tissues
-
Infiltration and activation of immune cells in the IVD results in amplification of the inflammatory responses and the release of neurotrophins
-
Inflammatory cytokines and neurotrophins promote the generation of pain through changes in nociceptive neuron ion channel activity, as well as apoptosis of these cells in the dorsal root ganglion
-
An enhanced understanding of the contribution of cytokines and immune cells to IVD degeneration, inflammation and nociception could enable the identification of novel potential therapeutic targets in symptomatic IVD disease
Abstract
Degeneration of the intervertebral discs (IVDs) is a major contributor to back, neck and radicular pain. IVD degeneration is characterized by increases in levels of the proinflammatory cytokines TNF, IL-1α, IL-1β, IL-6 and IL-17 secreted by the IVD cells; these cytokines promote extracellular matrix degradation, chemokine production and changes in IVD cell phenotype. The resulting imbalance in catabolic and anabolic responses leads to the degeneration of IVD tissues, as well as disc herniation and radicular pain. The release of chemokines from degenerating discs promotes the infiltration and activation of immune cells, further amplifying the inflammatory cascade. Leukocyte migration into the IVD is accompanied by the appearance of microvasculature tissue and nerve fibres. Furthermore, neurogenic factors, generated by both disc and immune cells, induce expression of pain-associated cation channels in the dorsal root ganglion. Depolarization of these ion channels is likely to promote discogenic and radicular pain, and reinforce the cytokine-mediated degenerative cascade. Taken together, an enhanced understanding of the contribution of cytokines and immune cells to these catabolic, angiogenic and nociceptive processes could provide new targets for the treatment of symptomatic disc disease. In this Review, the role of key inflammatory cytokines during each of the individual phases of degenerative disc disease, as well as the outcomes of major clinical studies aimed at blocking cytokine function, are discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Walker, B. F. The prevalence of low back pain: a systematic review of the literature from 1966 to 1998. J. Spinal Disord. 13, 205–217 (2000).
Martin, B. I. et al. Expenditures and health status among adults with back and neck problems. JAMA 299, 656–664 (2008).
Côté, P., Cassidy, J. D. & Carroll, L. The Saskatchewan Health and Back Pain Survey. The prevalence of neck pain and related disability in Saskatchewan adults. Spine (Phila Pa 1976) 23, 1689–1698 (1998).
Maniadakis, N. & Gray, A. The economic burden of back pain in the UK. Pain 84, 95–103 (2000).
Stewart, W. F., Ricci, J. A., Chee, E., Morganstein, D. & Lipton, R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA 290, 2443–2454 (2003).
Takatalo, J. et al. Does lumbar disc degeneration on MRI associate with low back symptom severity in young Finnish adults? Spine (Phila Pa 1976) 36, 2180–2189 (2011).
Livshits, G. et al. Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann. Rheum. Dis. 70, 1740–1745 (2011).
Roberts, S., Evans, H., Trivedi, J. & Menage, J. Histology and pathology of the human intervertebral disc. J. Bone Joint Surg. Am. 88, 10–14 (2006).
Kanayama, M., Togawa, D., Takahashi, C., Terai, T. & Hashimoto, T. Cross-sectional magnetic resonance imaging study of lumbar disc degeneration in 200 healthy individuals. J. Neurosurg. Spine 11, 501–507 (2009).
Cheung, K. M. et al. Prevalence and pattern of lumber magnetic resonance changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 34, 934–940 (2009).
Battié, M. C. et al. The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J. 9, 47–59 (2009).
Adams, M. A., Freeman, B. J., Morrison, H. P., Nelson, I. W. & Dolan, P. Mechanical initiation of intervertebral disc degeneration. Spine (Phila Pa 1976) 25, 1625–1636 (2000).
Wang, D. et al. Spine degeneration in a murine model of chronic human tobacco smokers. Osteoarthritis Cartilage 20, 896–905 (2012).
Stirling, A., Worthington, T., Rafiq, M., Lambert, P. A. & Elliott, T. S. Association between sciatica and Propionibacterium acnes. Lancet 357, 2024–2025 (2001).
Yamamoto, J. et al. Fas ligand plays an important role for the production of pro-inflammatory cytokines in intervertebral disc nucleus pulposus cells. J. Orthop. Res. 31, 608–615 (2013).
Rand, N., Reichert, F., Floman, Y. & Rotshenker, S. Murine nucleus pulposus-derived cells secrete interleukins-1-β, -6, and -10 and granulocyte-macrophage colony-stimulating factor in cell culture. Spine (Phila Pa 1976) 22, 2598–2601 (1997).
Kepler, C. K. et al. Substance P stimulates production of inflammatory cytokines in human disc cells. Spine (Phila Pa 1976) http://dx.doi.org/10.1097/BRS.0b013e3182a42bc22013.
Purmessur, D. et al. A role for TNFα in intervertebral disc degeneration: a non-recoverable catabolic shift. Biochem. Biophys. Res. Commun. 433, 151–156 (2013).
Shen, C., Yan, J., Jiang, L. S. & Dai, L. Y. Autophagy in rat annulus fibrosus cells: evidence and possible implications. Arthritis Res. Ther. 13, R132 (2011).
Le Maitre, C. L., Freemont, A. J. & Hoyland, J. A. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther. 7, R732–R745 (2005).
Le Maitre, C. L., Hoyland, J. A. & Freemont, A. J. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1β and TNFα expression profile. Arthritis Res. Ther. 9, R77 (2007).
Séguin, C. A., Pilliar, R. M., Roughley, P. J. & Kandel, R. A. Tumor necrosis factor-α modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976) 30, 1940–1948 (2005).
Shamji, M. F. et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 62, 1974–1982 (2010).
Cuéllar, J. M. et al. Cytokine expression in the epidural space: a model of noncompressive disc herniation-induced inflammation. Spine (Phila Pa 1976) 38, 17–23 (2013).
Hayashi, S. et al. TNF-α in nucleus pulposus induces sensory nerve growth: a study of the mechanism of discogenic low back pain using TNF-α-deficient mice. Spine (Phila Pa 1976) 33, 1542–1546 (2008).
Murata, Y. et al. Changes in pain behavior and histologic changes caused by application of tumor necrosis factor-alpha to the dorsal root ganglion in rats. Spine (Phila Pa 1976) 31, 530–535 (2006).
Wang, J. et al. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J. Biol. Chem. 286, 39738–39749 (2011).
Le Maitre, C. L., Hoyland, J. A. & Freemont, A. J. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis Res. Ther. 9, R83 (2007).
Pockert, A. J. et al. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 60, 482–491 (2009).
Bachmeier, B. E. et al. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur. Spine J. 18, 1573–1586 (2009).
Kokubo, Y. et al. Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J. Neurosurg. Spine. 9, 285–295 (2008).
Akyol, S., Eraslan. B. S., Etyemez, H., Tanriverdi, T. & Hanci, M. Catabolic cytokine expressions in patients with degenerative disc disease. Turk. Neurosurg. 20, 492–499 (2010).
Vernon-Roberts, B., Moore, R. J. & Fraser, R. D. The natural history of age-related disc degeneration: the pathology and sequelae of tears. Spine (Phila Pa 1976) 32, 2797–2804 (2007).
Melrose, J., Roberts, S., Smith, S., Menage, J. & Ghosh, P. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine (Phila Pa 1976) 27, 1278–1285 (2002).
Freemont, A. J. et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J. Pathol. 197, 286–292 (2002).
Ohtori, S. et al. Up-regulation of acid-sensing ion channel 3 in dorsal root ganglion neurons following application of nucleus pulposus on nerve root in rats. Spine (Phila Pa 1976) 31, 2048–2052 (2006).
Mamet, J., Lazdunski, M. & Voilley, N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J. Biol. Chem. 278, 48907–48913 (2003).
Zhang, X., Huang, J. & McNaughton, P. A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 24, 4211–4223 (2005).
Black, R. A. et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385, 729–733 (1997).
Cabal-Hierro, L. & Lazo, P. S. Signal transduction by tumor necrosis factor receptors. Cell Signal. 24, 1297–1305 (2012).
Silke, J. The regulation of TNF signalling: what a tangled web we weave. Curr. Opin. Immunol. 23, 620–626 (2011).
Weber, A., Wasiliew, P. & Kracht, M. Interleukin-1 (IL-1) pathway. Sci. Signal. 3, cm1 (2010).
Gabay, C., Lamacchia, C. & Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 6, 232–241 (2010).
Kepler, C. K. et al. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL-1β in painful human intervertebral discs. Spine (Phila Pa 1976) 38, 873–880 (2013).
Phillips, K. L., Jordan-Mahy, N., Nicklin, M. J. & Le Maitre, C. L. Interleukin-1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2012-202266.
Andrade, P. et al. Tumor necrosis factor-α levels correlate with postoperative pain severity in lumbar disc hernia patients: opposite clinical effects between tumor necrosis factor receptor 1 and 2. Pain 152, 2645–2652 (2011).
Ponnappan, R. K. et al. An organ culture system to model early degenerative changes of the intervertebral disc. Arthritis Res. Ther. 13, R171 (2011).
Oda, H. et al. Degeneration of intervertebral discs due to smoking: experimental assessment in a rat-smoking model. J. Orthop. Sci. 9, 135–141 (2004).
Walter, B. A. et al. Complex loading affects intervertebral disc mechanics and biology. Osteoarthritis Cartilage 19, 1011–1018 (2011).
Ulrich, J. A., Liebenberg, E. C., Thuillier, D. U. & Lotz, J. C. ISSLS prize winner: repeated disc injury causes persistent inflammation. Spine (Phila Pa 1976) 32, 2812–2819 (2007).
Rajan, N. et al. Toll-like receptor 4 (TLR4) expression and stimulation in a model of intervertebral disc inflammation and degeneration. Spine (Phila Pa 1976) http://dx.doi.org/10.1097/BRS.0b013e31826b71f4.
Tian, Y. et al. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-κB. Am. J. Pathol. http://dx.doi.org/10.1016/j.ajpath.2013.02.037.
Séguin, C. A., Pilliar, R. M., Madri, J. A. & Kandel, R. A. TNF-α induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine (Phila Pa 1976) 33, 356–365 (2008).
Echtermeyer, F. et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat. Med. 15, 1072–1076 (2009).
Séguin, C. A., Bojarski, M., Pilliar, R. M., Roughley, P. J. & Kandel, R. A. Differential regulation of matrix degrading enzymes in a TNFα-induced model of nucleus pulposus tissue degeneration. Matrix Biol. 25, 409–418 (2006).
Patel, K. P. et al. Aggrecanases and aggrecanase-generated fragments in the human intervertebral disc at early and advanced stages of disc degeneration. Spine (Phila Pa 1976) 32, 2596–2603 (2007).
Seki, S. et al. Effect of small interference RNA (siRNA) for ADAMTS5 on intervertebral disc degeneration in the rabbit anular needle-puncture model. Arthritis Res. Ther. 11, R166 (2009).
Fujita, N. et al. Expression of prolyl hydroxylases (PHDs) is selectively controlled by HIF-1 and HIF-2 proteins in nucleus pulposus cells of the intervertebral disc: distinct roles of PHD2 and PHD3 proteins in controlling HIF-1α activity in hypoxia. J. Biol. Chem. 287, 16975–16986 (2012).
Fujita, N. et al. Prolyl hydroxylase 3 (PHD3) modulates catabolic effects of tumor necrosis factor-α (TNF-α) on cells of the nucleus pulposus through co-activation of nuclear factor κB (NF-κB)/p65 signaling. J. Biol. Chem. 287, 39942–39953 (2012).
Kawaguchi, S. et al. Chemokine profile of herniated intervertebral discs infiltrated with monocytes and macrophages. Spine (Phila Pa 1976) 27, 1511–1516 (2002).
Wang, J. et al. Tumor necrosis factor α- and interleukin-1β-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 65, 832–842 (2013).
Ahn, S. H. et al. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine (Phila Pa 1976) 27, 911–917 (2002).
Takada, T. et al. Intervertebral disc and macrophage interaction induces mechanical hyperalgesia and cytokine production in a herniated disc model in rats. Arthritis Rheum. 64, 2601–2610 (2012).
Hiyama, A. et al. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: implications in degenerative disc disease. Arthritis Rheum. 63, 1355–1364 (2011).
Wang, H. et al. Inflammatory cytokines induce notch signaling in nucleus pulposus cells: implications in intervertebral disc degeneration. J. Biol. Chem. 288, 16761–16774 (2013).
Scheller, J., Chalaris, A., Schmidt-Arras, D. & Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888 (2011).
Andrade, P. et al. Elevated IL-1β and IL-6 levels in lumbar herniated discs in patients with sciatic pain. Eur. Spine J. 22, 714–720 (2013).
Studer, R. K., Vo, N., Sowa, G., Ondeck, C. & Kang, J. Human nucleus pulposus cells react to IL-6: independent actions and amplification of response to IL-1 and TNF-α. Spine (Phila Pa 1976) 36, 593–599 (2011).
Murata, Y. et al. Local application of interleukin-6 to the dorsal root ganglion induces tumor necrosis factor-α in the dorsal root ganglion and results in apoptosis of the dorsal root ganglion cells. Spine (Phila Pa 1976) 36, 926–932 (2011).
Murata, Y., Nannmark, U., Rydevik, B., Takahashi, K. & Olmarker, K. The role of tumor necrosis factor-α in apoptosis of dorsal root ganglion cells induced by herniated nucleus pulposus in rats. Spine (Phila Pa 1976) 33, 155–162 (2008).
Wei, X. H. et al. The up-regulation of IL-6 in DRG and spinal dorsal horn contributes to neuropathic pain following L5 ventral root transection. Exp. Neurol. 241, 159–168 (2013).
Noponen-Hietala, N. et al. Genetic variations in IL6 associate with intervertebral disc disease characterized by sciatica. Pain 114, 186–194 (2005).
Kelempisioti, A. et al. Genetic susceptibility of intervertebral disc degeneration among young Finnish adults. BMC Med. Genet. 12, 153 (2011).
Gaffen, S. L. Recent advances in the IL-17 cytokine family. Curr. Opin. Immunol. 23, 613–619 (2011).
Gruber, H. E, Hoelscher, G. L., Ingram, J. A., Norton, H. J. & Hanley, E. N. Jr. Increased IL-17 expression in degenerated human discs and increased production in cultured annulus cells exposed to IL-1β and TNF-α. Biotech. Histochem. http://dx.doi.org/10.3109/10520295.2013.783235.
Kenna, T. J. & Brown, M. A. The role of IL-17-secreting mast cells in inflammatory joint disease. Nat. Rev. Rheumatol. http://dx.doi.org/10.1038/nrrheum.2012.205.
Schroder, K., Hertzog, P. J., Ravasi, T. & Hume, D. A. Interferon-γ: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75, 163–189 (2004).
Sadir, R., Forest, E. & Lortat-Jacob, H. The heparan sulfate binding sequence of interferon-γ increased the on rate of the interferon-γ-interferon-γreceptor complex formation. J. Biol. Chem. 273, 10919–10925 (1998).
Kim, C. F. & Moalem-Taylor, G. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J. Pain. 12, 370–383 (2011).
Gabr, M. A. et al. Interleukin-17 synergizes with IFNγ or TNFα to promote inflammatory mediator release and intercellular adhesion molecule-1 (ICAM-1) expression in human intervertebral disc cells. J. Orthop. Res. 29, 1–7 (2011).
Park, J. B., Chang, H. & Kim, Y. S. The pattern of interleukin-12 and T-helper types 1 and 2 cytokine expression in herniated lumbar disc tissue. Spine (Phila Pa 1976) 27, 2125–2128 (2002).
Cuellar, J. M. et al. Cytokine evaluation in individuals with low back pain using discographic lavage. Spine J. 10, 212–218 (2010).
Tian, P., Ma, X. L., Wang, T., Ma, J. X. & Yang, X. Correlation between radiculalgia and counts of T lymphocyte subsets in the peripheral blood of patients with lumbar disc herniation. Orthop. Surg. 1, 317–321 (2009).
Ma, X. L., Tian, P., Wang, T. & Ma, J. X. A study of the relationship between type of lumbar disc herniation, straight leg raising test and peripheral T lymphocytes. Orthop. Surg. 2, 52–57 (2010).
Johnson, W. E. et al. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 46, 2658–2664 (2002).
Tolofari, S. K., Richardson, S. M., Freemont, A. J. & Hoyland, J. A. Expression of semaphorin 3A and its receptors in the human intervertebral disc: potential role in regulating neural ingrowth in the degenerate intervertebral disc. Arthritis Res. Ther. 12, R1 (2010).
Uchiyama, Y. et al. Expression of acid-sensing ion channel 3 (ASIC3) in nucleus pulposus cells of the intervertebral disc is regulated by p75NTR and ERK signaling. J. Bone Miner. Res. 22, 1996–2006 (2007).
Purmessur, D., Freemont, A. J. & Hoyland, J. A. Expression and regulation of neurotrophins in the nondegenerate and degenerate human intervertebral disc. Arthritis Res. Ther. 10, R99 (2008).
Abe, Y. et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine (Phila Pa 1976) 32, 635–642 (2007).
Gruber, H. E., Hoelscher, G. L., Bethea, S. & Hanley, E. N. Jr. Interleukin 1-β upregulates brain-derived neurotrophic factor, neurotrophin 3 and neuropilin 2 gene expression and NGF production in annulus cells. Biotech. Histochem. 87, 506–511 (2012).
Ebbinghaus, M. et al. The role of interleukin-1β in arthritic pain: main involvement in thermal, but not mechanical, hyperalgesia in rat antigen-induced arthritis. Arthritis Rheum. 64, 3897–3907 (2012).
Ohtori, S., Takahashi, K. & Moriya, H. Existence of brain-derived neurotrophic factor and vanilloid receptor subtype 1 immunoreactive sensory DRG neurons innervating L5/6 intervertebral discs in rats. J. Orthop. Sci. 8, 84–87 (2003).
Ashton, I. K., Roberts, S., Jaffray, D. C., Polak, J. M. & Eisenstein, S. M. Neuropeptides in the human intervertebral disc. J. Orthop. Res. 12, 186–192 (1994).
Brown, M. F. et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J. Bone Joint Surg. Br. 79, 147–153 (1997).
Ohtori, S. et al. Substance P and calcitonin gene-related peptide immunoreactive sensory DRG neurons innervating the lumbar intervertebral discs in rats. Ann. Anat. 184, 235–240 (2002).
García-Cosamalón, J. et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain? J. Anat. 217, 1–15 (2010).
Ohtori, S. et al. Epidural administration of spinal nerves with the tumor necrosis factor-α inhibitor, etanercept, compared with dexamethasone for treatment of sciatica in patients with lumbar spinal stenosis: a prospective randomized study. Spine (Phila Pa 1976) 37, 439–444 (2012).
Ohtori, S. et al. Efficacy of epidural administration of anti-interleukin-6 receptor antibody onto spinal nerve for treatment of sciatica. Eur. Spine J. 21, 2079–2084 (2012).
Genevay, S. et al. Adalimumab in severe and acute sciatica: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 62, 2339–2346 (2010).
Genevay, S. et al. Adalimumab in acute sciatica reduces the long-term need for surgery: a 3-year follow-up of a randomised double-blind placebo-controlled trial. Ann. Rheum. Dis. 71, 560–562 (2012).
Genevay, S., Stingelin, S. & Gabay, C. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann. Rheum. Dis. 63, 1120–1123 (2004).
Cohen, S. P., Bogduk, N. & Dragovich, A. Randomized, double-blind, placebo-controlled, dose-response, and preclinical safety study of transforaminal epidural etanercept for the treatment of sciatica. Anesthesiology 110, 1116–1126 (2009).
Okoro, T., Tafazal, S. I., Longworth, S. & Sell, P. J. Tumor necrosis α-blocking agent (etanercept): a triple blind randomized controlled trial of its use in treatment of sciatica. J. Spinal Disord. Tech. 23, 74–77 (2010).
Cohen, S. P. et al. Epidural steroids, etanercept, or saline in subacute sciatica: a multicenter, randomized trial. Ann. Intern. Med. 156, 551–559 (2012).
Korhonen, T. et al. The treatment of disc-herniation-induced sciatica with infliximab: one-year follow-up results of FIRST II, a randomized controlled trial. Spine (Phila Pa 1976) 31, 2759–2766 (2006).
Nadeau, S. et al. Functional recovery after peripheral nerve injury is dependent on the pro-inflammatory cytokines IL-1β and TNF: implications for neuropathic pain. J. Neurosci. 31, 12533–12542 (2011).
Smith, L. J. et al. Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine challenge following long-term agarose culture. Eur. Cell Mater. 22, 291–301 (2011).
Gorth, D. J. et al. IL-1ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1β-mediated degradation of nucleus pulposus in vitro. Arthritis Res. Ther. 14, R179 (2012).
Rothman, S. M., Huang, Z., Lee, K. E., Weisshaar, C. L. & Winkelstein, B. A. Cytokine mRNA expression in painful radiculopathy. J. Pain. 10, 90–99 (2009).
Rothman, S. M. & Winkelstein, B. A. Cytokine antagonism reduces pain and modulates spinal astrocytic reactivity after cervical nerve root compression. Ann. Biomed. Eng. 38, 2563–2576 (2010).
Kim, J. S. et al. Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. J. Cell Physiol. http://dx.doi.org/10.1002/jcp.24350.
Klawitter, M. et al. Triptolide exhibits anti-inflammatory, anti-catabolic as well as anabolic effects and suppresses TLR expression and MAPK activity in IL-1β treated human intervertebral disc cells. Eur. Spine J. 21, S850–S859 (2012).
Ellman, M. B. et al. Toll-like receptor adaptor signaling molecule MyD88 on intervertebral disk homeostasis: in vitro, ex vivo studies. Gene 505, 283–290 (2012).
Winkelstein, B. A., Rutkowski, M. D., Sweitzer, S. M., Pahl, J. L. & DeLeo, J. A. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J. Comp. Neurol. 439, 127–139 (2001).
Sinclair, S. M. et al. Attenuation of inflammatory events in human intervertebral disc cells with a tumor necrosis factor antagonist. Spine (Phila Pa 1976) 36, 1190–1196 (2011).
Schafers, M., Svensson, C. I., Sommer, C. & Sorkin, L. S. Tumor necrosis factor-α induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 23, 2517–2521 (2003).
Allen, K. D. et al. Kinematic and dynamic gait compensations in a rat model of lumbar radiculopathy and the effects of tumor necrosis factor-α antagonism. Arthritis Res. Ther. 13, R137 (2011).
Zanella, J. M. et al. Effect of etanercept, a tumor necrosis factor-α inhibitor, on neuropathic pain in the rat chronic constriction injury model. Spine (Phila Pa 1976) 33, 227–234 (2008).
Nasto, L. A. et al. Inhibition of NF-κB activity ameliorates age-associated disc degeneration in a mouse model of accelerated aging. Spine (Phila Pa 1976) 37, 1819–1825 (2012).
Suzuki, M. et al. Nuclear factor-κ B decoy suppresses nerve injury and improves mechanical allodynia and thermal hyperalgesia in a rat lumbar disc herniation model. Eur. Spine J. 18, 1001–1007 (2009).
Acknowledgements
The work of the authors is supported by US NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) awards AR050087 and AR055655. The authors wish to thank C. K. Kepler (Rothman Institute and Thomas Jefferson University, Philadelpia, PA, USA) for providing the MRI image of a herniated disc.
Author information
Authors and Affiliations
Contributions
Both authors made substantial contributions to all stages of the preparation of this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Risbud, M., Shapiro, I. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 10, 44–56 (2014). https://doi.org/10.1038/nrrheum.2013.160
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.160
This article is cited by
-
Serglycin secreted by late-stage nucleus pulposus cells is a biomarker of intervertebral disc degeneration
Nature Communications (2024)
-
Ginsenoside Rg1 relieves rat intervertebral disc degeneration and inhibits IL-1β-induced nucleus pulposus cell apoptosis and inflammation via NF-κB signaling pathway
In Vitro Cellular & Developmental Biology - Animal (2024)
-
Degenerated nucleus pulposus cells derived exosome carrying miR-27a-3p aggravates intervertebral disc degeneration by inducing M1 polarization of macrophages
Journal of Nanobiotechnology (2023)
-
BMP7 ameliorates intervertebral disc degeneration in type 1 diabetic rats by inhibiting pyroptosis of nucleus pulposus cells and NLRP3 inflammasome activity
Molecular Medicine (2023)
-
Codelivery of TGF-β1 and anti-miR-141 by PLGA microspheres inhibits progression of intervertebral disc degeneration
Journal of Orthopaedic Surgery and Research (2023)