Abstract
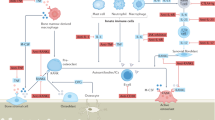
Bone erosion is a central feature of rheumatoid arthritis and is associated with disease severity and poor functional outcome. Erosion of periarticular cortical bone, the typical feature observed on plain radiographs in patients with rheumatoid arthritis, results from excessive local bone resorption and inadequate bone formation. The main triggers of articular bone erosion are synovitis, including the production of proinflammatory cytokines and receptor activator of nuclear factor κB ligand (RANKL), as well as antibodies directed against citrullinated proteins. Indeed, both cytokines and autoantibodies stimulate the differentiation of bone-resorbing osteoclasts, thereby stimulating local bone resorption. Although current antirheumatic therapy inhibits both bone erosion and inflammation, repair of existing bone lesions, albeit physiologically feasible, occurs rarely. Lack of repair is due, at least in part, to active suppression of bone formation by proinflammatory cytokines. This Review summarizes the substantial progress that has been made in understanding the pathophysiology of bone erosions and discusses the improvements in the diagnosis, monitoring and treatment of such lesions.
Key Points
-
Articular bone erosions are a central clinical feature of rheumatoid arthritis
-
Imaging techniques enable early detection of bone erosions and provide insights into disease pathogenesis
-
Bone erosion is a result of enhanced osteoclast differentiation and inhibition of osteoblast-mediated bone repair
-
Autoantibodies and cytokines, including proinflammatory cytokines and receptor activator of nuclear factor κB ligand, are the major precipitating factors in bone erosion in rheumatoid arthritis
-
Antirheumatic therapies block progression of bone erosion by mitigating synovial inflammation and restoring bone balance
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Karsenty, G., Kronenberg, H. M. & Settembre, C. Genetic control of bone formation. Annu. Rev. Cell. Dev. Biol. 25, 629–648 (2009).
Teitelbaum, S. L. & Ross, F. P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649 (2003).
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003).
Baker, W. M. The formation of abnormal synovial cysts in the connection with the joints. St Bartolomews Hospital Reports 21, 177–190 (1855).
Weichselbaum, A. Die feineren Veränderungen des Gelenkknorpels bei fungöser Synovitis und Karies der Gelenkenden [German] Archiv. Pathol. Anat. Physiol. Klin. Med. 73, 461–475 (1878).
Takayanagi, H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 7, 292–304 (2007).
Arron, J. R. & Choi, Y. Osteoimmunology: bone versus immune system. Nature 408, 535–536 (2000).
Schett, G. & David, J. P. The multiple faces of autoimmune-mediated bone loss. Nat. Rev. Endocrinol. 6, 698–706 (2010).
Schett, G. Saag, K. G. & Bijlsma, J. W. From bone biology to clinical outcome: state of the art and future perspectives. Ann. Rheum. Dis. 69, 1415–1419 (2010).
Sharp, J. T., Lidsky, M. D., Collins, L. C. & Moreland, J. Methods of scoring the progression of radiologic changes in rheumatoid arthritis. Correlation of radiologic, clinical and laboratory abnormalities. Arthritis Rheum. 14, 706–720 (1971).
Aletaha, D. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/ European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 69, 1580–1588 (2010).
Ødegård, S. et al. Association of early radiographic damage with impaired physical function in rheumatoid arthritis: a ten-year, longitudinal observational study in 238 patients. Arthritis Rheum. 54, 68–75 (2006).
Scott, D. L. et al. The links between joint damage and disability in rheumatoid arthritis. Rheumatology (Oxford) 39, 122–132 (2000).
Welsing, P. M., van Gestel, A. M., Swinkels, H. L., Kiemeney, L. A. & van Riel, P. L. The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 44, 2009–2017 (2001).
McInnes, I. & Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Immunol. 7, 429–442 (2007).
Stach, C.M. et al. Periarticular bone structure in rheumatoid arthritis patients and healthy individuals assessed by high-resolution computed tomography. Arthritis Rheum. 62, 330–339 (2010).
Døhn, U. M. et al. Rheumatoid arthritis bone erosion volumes on CT and MRI: reliability and correlations with erosion scores on CT, MRI and radiography. Ann. Rheum. Dis. 66, 1388–1392 (2007).
Wakefield, R. J. et al. The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum. 43, 2762–2770 (2000).
McGonagle, D., Tan, A. L., Møller Døhn, U., Ostergaard, M. & Benjamin, M. Microanatomic studies to define predictive factors for the topography of periarticular erosion formation in inflammatory arthritis. Arthritis Rheum. 60, 1042–1051 (2009).
Martel, W., Hayes, J. T. & Duff, I. F. The pattern of bone erosion in the hand and wrist in rheumatoid arthritis. Radiology 84, 204–214 (1965).
Ejbjerg, B. et al. Magnetic resonance imaging of wrist and finger joints in healthy subjects occasionally shows changes resembling erosions and synovitis as seen in rheumatoid arthritis. Arthritis Rheum. 50, 1097–1106 (2004).
Tan, A. L. et al. Role of metacarpophalangeal joint anatomic factors in the distribution of synovitis and bone erosion in early rheumatoid arthritis. Arthritis Rheum. 48, 1214–1222 (2003).
Hayer, S. et al. Tenosynovitis and osteoclast formation as the initial preclinical changes in a murine model of inflammatory arthritis. Arthritis Rheum. 56, 79–88 (2007).
Marinova-Mutafchieva, L., Williams, R. O., Funa, K., Maini, R. N. & Zvaifler, N. J. Inflammation is preceded by tumor necrosis factor-dependent infiltration of mesenchymal cells in experimental arthritis. Arthritis Rheum. 46, 507–513 (2002).
Schett, G. et al. Analysis of the kinetics of osteoclastogenesis in arthritic rats. Arthritis Rheum. 52, 3192–3201 (2005).
Tournis, S. et al. Effect of rheumatoid arthritis on volumetric bone mineral density and bone geometry, assessed by peripheral quantitative computed tomography in postmenopausal women treated with bisphosphonates. J. Rheumatol. 39, 1215–1220 (2012).
Aeberli, D. et al. Reduced trabecular bone mineral density and cortical thickness accompanied by increased outer bone circumference in metacarpal bone of rheumatoid arthritis patients: a cross-sectional study. Arthritis Res. Ther. 12, R119 (2010).
Sharp, J. T. et al. Denosumab prevents metacarpal shaft cortical bone loss in patients with erosive rheumatoid arthritis. Arthritis Care Res. (Hoboken) 62, 537–544 (2010).
Hoff, M. et al. Cortical hand bone loss after 1 year in early rheumatoid arthritis predicts radiographic hand joint damage at 5-year and 10-year follow-up. Ann. Rheum. Dis. 68, 324–329 (2009).
Haugeberg, G. et al. Hand cortical bone mass and its associations with radiographic joint damage and fractures in 50–70 year old female patients with rheumatoid arthritis: cross sectional Oslo-Truro-Amsterdam (OSTRA) collaborative study. Ann. Rheum. Dis. 63, 1331–1334 (2004).
Van der Heijde, D. M. Joint erosions and patients with early rheumatoid arthritis. Br. J. Rheumatol. 34, 74–78 (1995).
Machold, K. P. et al. Very recent onset rheumatoid arthritis: clinical and serological patient characteristics associated with radiographic progression over the first years of disease. Rheumatology (Oxford) 46, 342–349 (2007).
Güler-Yüksel, M. et al. Changes in hand and generalised bone mineral density in patients with recent-onset rheumatoid arthritis. Ann. Rheum. Dis. 68, 330–336 (2009).
Solomon, D. H. et al. The relationship between focal erosions and generalized osteoporosis in postmenopausal women with rheumatoid arthritis. Arthritis Rheum. 60, 1624–1631 (2009).
Pye, S. R. et al. Disease activity and severity in early inflammatory arthritis predict hand cortical bone loss. Rheumatology (Oxford) 49, 1943–1948 (2010).
Møller Døhn, U. et al. No overall progression and occasional repair of erosions despite persistent inflammation in adalimumab-treated rheumatoid arthritis patients: results from a longitudinal comparative MRI, ultrasonography, CT and radiography study. Ann. Rheum. Dis. 70, 252–258 (2011).
Finzel, S. et al. A detailed comparative study of high-resolution ultrasound and micro-computed tomography for detection of arthritic bone erosions. Arthritis Rheum. 63, 1231–1236 (2011).
Haavardsholm, E. A., Bøyesen, P., Østergaard, M., Schildvold, A. & Kvien, T. K. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann. Rheum. Dis. 67, 794–800 (2008).
McQueen, F. M. et al. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 48, 1814–1827 (2003).
Geusens, P. & Lems, W. F. Osteoimmunology and osteoporosis. Arthritis Res. Ther. 13, 242 (2011).
Finzel, S., Englbrecht, M., Engelke, K., Stach, C. & Schett, G. A comparative study of periarticular bone lesions in rheumatoid arthritis and psoriatic arthritis. Ann. Rheum. Dis. 70, 122–127 (2011).
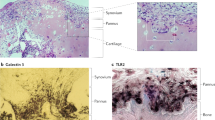
Bromley, M. & Woolley, D. E. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum. 27, 968–975 (1984).
Leisen, J. C. D. H., Riddle, J. M. & Pitchford, W. C. The erosive front: a topographic study of the junction between the pannus and the subchondral plate in the macerated rheumatoid metacarpal head. J. Rheumatol. 15, 17–22 (1988).
Gravallese, E. M. et al. Identification of cell types responsible for bone resorption in rheumatoid arthritis and juvenile rheumatoid arthritis. Am. J. Pathol. 152, 943–951 (1998).
Kong, Y. Y. et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402, 304–309 (1999).
Romas, E. et al. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am. J. Pathol. 161, 1419–1427 (2002).
Lubberts, E. et al. Increase in expression of receptor activator of nuclear factor κB at sites of bone erosion correlates with progression of inflammation in evolving collagen-induced arthritis. Arthritis Rheum. 46, 3055–3064 (2002).
Pettit, A. R. et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am. J. Pathol. 159, 1689–1699 (2001).
Redlich, K. et al. Osteoclasts are essential for TNF-α-mediated joint destruction. J. Clin. Invest. 110, 1419–1427 (2002).
Redlich, K. et al. Tumor necrosis factor α-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum. 46, 785–792 (2002).
Yoshida, H. et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345, 442–444 (1990).
Firestein, G. S. et al. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J. Exp. Med. 168, 1573–1586 (1988).
Wong, B. R. et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J. Biol. Chem. 272, 25190–25194 (1997).
Yasuda, H. et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl Acad. Sci. USA 95, 3597–3602 (1998).
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Kong, Y.-Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315–323 (1999).
Gravallese, E. M. et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 43, 250–258 (2000).
Shigeyama, Y. et al. Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum. 43, 2523–2530 (2000).
Li, P. et al. RANK signaling is not required for TNFα-mediated increase in CD11hi osteoclast precursors but is essential for mature osteoclast formation in TNFα-mediated inflammatory arthritis. J. Bone Miner. Res. 19, 207–213 (2004).
Ritchlin, C. T., Haas-Smith, S. A., Li, P., Hicks, D. G. & Schwarz, E. M. Mechanisms of TNF-α- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J. Clin. Invest. 111, 821–831 (2003).
Hermann, S. et al. OSCAR—a key co-stimulation molecule for osteoclasts is induced in patients with rheumatoid arthritis. Arthritis Rheum. 58, 3041–3050 (2008).
Ohno, H. et al. The orally-active and selective c-Fms tyrosine kinase inhibitor Ki20227 inhibits disease progression in a collagen-induced arthritis mouse model. Eur. J. Immunol. 38, 283–291 (2008).
Cohen, S. B. et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 58, 1299–1309 (2008).
Visser, H., le Cessie, S., Vos, K., Breedveld, F. C. & Hazes, J. M. How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum. 46, 357–365 (2002).
Kastbom, A., Strandberg, G., Lindroos, A. & Skogh, T. Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann. Rheum. Dis. 63, 1085–1089 (2004).
Meyer, O. et al. Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann. Rheum. Dis. 62, 120–126 (2003).
Shi, J. et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl Acad. Sci. USA 108, 17372–17377 (2011).
Harre, U. et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 122, 1791–1802 (2012).
Wu, Y., Humphrey, M. B. & Nakamura, M. C. Osteoclasts—the innate immune cells of the bone. Autoimmunity 41, 183–194 (2008).
Ji, D. et al. Inhibition of RANK expression and osteoclastogenesis by TLRs and IFN-γ in human osteoclast precursors. J. Immunol. 183, 7223–7233 (2009).
Sato, K. et al. TH17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203, 2673–2682 (2006).
Zaiss, M. M. et al. Regulatory T cells protect from local and systemic bone destruction in arthritis. J. Immunol. 184, 7238–7246 (2010).
Zaiss, M. M. et al. Increased bone density and resistance to ovariectomy-induced bone loss in FoxP3-transgenic mice based on impaired osteoclast differentiation. Arthritis Rheum. 62, 2328–2338 (2010).
Axmann, R. et al. CTLA-4 directly inhibits osteoclast formation. Ann. Rheum. Dis. 67, 1603–1609 (2008).
Zaiss, M. M. et al. TREG cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 56, 4104–4112 (2007).
Bakker, M. F. et al. Utrecht Rheumatoid Arthritis Cohort Study Group. Low-dose prednisone inclusion in a methotrexate-based, tight control strategy for early rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 156, 329–339 (2012).
Grigor, C. et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 364, 263–269 (2004).
Lam, J. et al. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 106, 1481–1488 (2000).
Boyce, B. F. et al. Effects of interleukin-1 on bone turnover in normal mice. Endocrinology 125, 1142–1150 (1989).
Zwerina, J. et al. TNF-induced structural joint damage is mediated by IL-1. Proc. Natl Acad. Sci. USA 104, 11742–11747 (2007).
Ishimi, Y. M. C. et al. IL-6 is produced by osteoblasts and induces bone resorption. J. Immunol. 145, 3297–3303 (1990).
Axmann, R. et al. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 60, 2747–2756 (2009).
Poli, V. et al. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. EMBO J. 13, 1189–1196 (1994).
Jansen, L. M., van der Horst-Bruinsma, I. E., van Schaardenburg, D., Bezemer, P. D. & Dijkmans, B. A. Predictors of radiographic joint damage in patients with early rheumatoid arthritis. Ann. Rheum. Dis. 60, 924–927 (2001).
Kraan, M. C. et al. Asymptomatic synovitis precedes clinically manifest arthritis. Arthritis Rheum. 41, 1481–1488 (1998).
Van de Sande, M. G. et al. Different stages of rheumatoid arthritis: features of the synovium in the preclinical phase. Ann. Rheum. Dis. 70, 772–777 (2011).
Rich, E., Moreland, L. W. & Alarcón, G.S. Paucity of radiographic progression in rheumatoid arthritis treated with methotrexate as the first disease modifying antirheumatic drug. J. Rheumatol. 26, 259–261 (1999).
Schett, G., Stach, C., Zwerina, J., Voll, R. & Manger, B. How antirheumatic drugs protect joints from damage in rheumatoid arthritis. Arthritis Rheum. 58, 2936–2948 (2008).
Cohen, G. et al. Radiological damage in patients with rheumatoid arthritis on sustained remission. Ann. Rheum. Dis. 66, 358–363 (2007).
Molenaar, E. T. et al. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 50, 36–42 (2004).
Brown, A. K. et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 58, 2958–2967 (2008).
Vis, M. et al. Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFκB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 65, 1495–1499 (2006).
Pesu, M. et al. Therapeutic targeting of Janus kinases. Immunol. Rev. 223, 132–142 (2008).
van Vollenhoven, R. F. et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N. Engl. J. Med. 367, 508–519 (2012).
Fleischmann, R. et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N. Engl. J. Med. 367, 495–507 (2012).
Weinblatt, M. E. et al. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N. Engl. J. Med. 363, 1303–1312 (2010).
Mócsai, A. et al. The immunomodulatory adapter proteins DAP12 and Fc receptor γ-chain (FcRγ) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl Acad. Sci. USA 101, 6158–6163 (2004).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Jiang, Y. et al. A multicenter, double-blind, dose-ranging, randomized, placebo-controlled study of recombinant human interleukin-1 receptor antagonist in patients with rheumatoid arthritis: radiologic progression and correlation of Genant and Larsen scores. Arthritis Rheum. 43, 1001–1009 (2000).
Fossiez, F. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593–2603 (1996).
Jovanovic, D. V. et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages. J. Immunol. 160, 3513–3521 (1998).
Chabaud, M. et al. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42, 963–970 (1999).
Miossec, P., Korn, T. & Kuchroo, V. K. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 361, 888–889 (2009).
Kotake, S. et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103, 1345–1352 (1999).
Sato, K. et al. TH17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203, 2673–2682 (2006).
Zwerina, K. et al. Anti IL-17A therapy inhibits bone loss in TNF-α-mediated murine arthritis by modulation of the T-cell balance. Eur. J. Immunol. 42, 413–423 (2012).
Ogata, Y. et al. A novel role of IL-15 in the development of osteoclasts: inability to replace its activity with IL-2. J. Immunol. 162, 2754–2760 (1999).
Knevel, R. et al. Genetic variants in IL15 associate with progression of joint destruction in rheumatoid arthritis: a multicohort study. Ann. Rheum. Dis. 71 (Suppl. 1), A56–A57 (2012).
Zaiss, M. M. et al. IL-33 shifts the balance from osteoclast to alternatively activated macrophage differentiation and protects from TNFα-mediated bone loss. J. Immunol. 186, 6097–6105 (2011).
Quinn, J. M. et al. IL-23 inhibits osteoclastogenesis indirectly through lymphocytes and is required for the maintenance of bone mass in mice. J. Immunol. 181, 5720–5729 (2008).
Lukas, C., van der Heijde, D., Fatenejad, S. & Landewé, R. Repair of erosions occurs almost exclusively in damaged joints without swelling. Ann. Rheum. Dis. 69, 851–855 (2010).
Møller Døhn, U. et al. Erosive progression is minimal, but erosion healing rare, in patients with rheumatoid arthritis treated with adalimumab. A 1-year investigator-initiated follow-up study using high-resolution computed tomography as the primary outcome measure. Ann. Rheum. Dis. 68, 1585–1590 (2009).
Finzel, S. et al. Interleukin-6 receptor blockade induces limited repair of bone erosions in rheumatoid arthritis: a micro CT study. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2011-201075.
Finzel, S. et al. Repair of bone erosions in rheumatoid arthritis treated with tumour necrosis factor inhibitors is based on bone apposition at the base of the erosion. Ann. Rheum. Dis. 70, 1587–1593 (2011).
Walsh, N. C. et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J. Bone Miner. Res. 24, 1572–1585 (2009).
Diarra D, Stolina M, Polzer K. et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 13, 156–163 (2007).
Matzelle, M. M. et al. Resolution of inflammation induces osteoblast function and regulates the Wnt signaling pathway. Arthritis Rheum. 64, 1540–1550 (2012).
Redlich, K. et al. Repair of local bone erosions and reversal of systemic bone loss upon therapy with anti-tumor necrosis factor in combination with osteoprotegerin or parathyroid hormone in tumor necrosis factor-mediated arthritis. Am. J. Pathol. 164, 543–555 (2004).
Poole, K. E. et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 19, 1842–1844 (2005).
Semënov, M., Tamai, K. & He, X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 280, 26770–26775 (2005).
Acknowledgements
The work of G. Schett is supported by the Deutsche Forschungsgemeinschaft (SPP1468-IMMUNOBONE), the Bundesministerium f¨r Bildung und Forschung (BMBF; project ANCYLOSS) and the MASTERSWITCH project of the European Union and the IMI funded project BTCure. The work of E. Gravallese is supported by the NIH (R01 AR055952) and by the American College of Rheumatology Research and Education Foundation (Within our Reach: Finding a Cure for Rheumatoid Arthritis campaign).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
G. Schett declares no competing interests. E. Gravallese has acted as a consultant for Abbott Laboratories and has received grant/research support from Lilly.
Rights and permissions
About this article
Cite this article
Schett, G., Gravallese, E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 8, 656–664 (2012). https://doi.org/10.1038/nrrheum.2012.153
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.153
This article is cited by
-
Modulation of fracture healing by senescence-associated secretory phenotype (SASP): a narrative review of the current literature
European Journal of Medical Research (2024)
-
Eosinophils preserve bone homeostasis by inhibiting excessive osteoclast formation and activity via eosinophil peroxidase
Nature Communications (2024)
-
Bone density and fracture risk factors in ankylosing spondylitis: a meta-analysis
Osteoporosis International (2024)
-
HSP90 Exacerbates Bone Destruction in Rheumatoid Arthritis by Activating TRAF6/NFATc1 Signaling
Inflammation (2024)
-
Society of skeletal radiology position paper – recommendations for contrast use in musculoskeletal MRI: when is non-contrast imaging enough?
Skeletal Radiology (2024)