Abstract
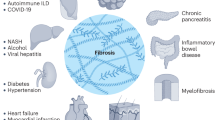
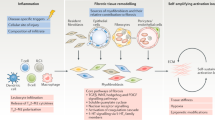
Fibrosis in multiple organs is a prominent pathological finding and distinguishing hallmark of systemic sclerosis (SSc). Findings during the past 5 years have contributed to a more complete understanding of the complex cellular and molecular underpinning of fibrosis in SSc. Fibroblasts, the principal effector cells, are activated in the profibrotic cellular milieu by cytokines and growth factors, developmental pathways, endothelin 1 and thrombin. Innate immune signaling via Toll-like receptors, matrix-generated biomechanical stress signaling via integrins, hypoxia and oxidative stress seem to be implicated in perpetuating the process. Beyond chronic fibroblast activation, fibrosis represents a failure to terminate tissue repair, coupled with an expanded population of mesenchymal cells originating from bone marrow and transdifferentiation of epithelial cells, endothelial cells and pericytes. In addition, studies have identified intrinsic alterations in SSc fibroblasts resulting from epigenetic changes, as well as altered microRNA expression that might underlie the cell-autonomous, persistent activation phenotype of these cells. Precise characterization of the deregulated extracellular and intracellular signaling pathways, mediators and cellular differentiation programs that contribute to fibrosis in SSc will facilitate the development of selective, targeted therapeutic strategies. Effective antifibrotic therapy will ultimately involve novel compounds and repurposing of drugs that are already approved for other indications.
Key Points
-
Fibrosis represents a deregulated and uncontrolled repair process that recapitulates features of embryonic development and normal wound healing
-
Fibrosis in systemic sclerosis (SSc) shares mechanisms that underlie organ-based fibrosing disorders such as idiopathic pulmonary fibrosis
-
Transforming growth factor β seems to be a master regulator of both physiological and pathological matrix remodeling, and might be responsible for maintaining the activated fibroblast phenotype in SSc
-
The Wnt–β-catenin, Hedgehog–Patched and Jagged–Notch pathways are important in embryological development, and seem to be aberrantly activated in some patients with SSc
-
Mesenchymal cells are chronically activated via self-amplifying loops, resulting in a vicious cycle of progressive fibrogenesis
-
Selective targeting of molecules and pathways involved in fibroblast activation, singly or in combination, offers new approaches to the treatment of fibrosis
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Varga, J. & Abraham, D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J. Clin. Invest. 117, 557–567 (2007).
Rosenbloom, J., Castro, S. V. & Jimenez, S. A. Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann. Intern. Med. 152, 159–166 (2010).
Denton, C. P., Black, C. M. & Abraham, D. J. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat. Clin. Pract. Rheumatol. 2, 134–144 (2006).
Wei, J., Bhattacharyya, S., Tourtellotte, W. G. & Varga, J. Fibrosis in systemic sclerosis: emerging concepts and implications for targeted therapy. Autoimmun. Rev. 10, 267–275 (2011).
Quillinan, N. P. & Denton, C. P. Disease-modifying treatment in systemic sclerosis: current status. Curr. Opin. Rheumatol. 21, 636–641 (2009).
Distler, J. & Distler, O. Novel treatment approaches to fibrosis in scleroderma. Rheum. Dis. Clin. North Am. 34, 145–159 (2008).
Trojanowska, M. & Varga, J. Molecular pathways as novel therapeutic targets in systemic sclerosis. Curr. Opin. Rheumatol. 19, 568–573 (2007).
van der Slot, A. J. et al. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol. 23, 251–257 (2004).
Higgins, D. F. et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 117, 3810–3820 (2007).
Gardner, H. et al. Gene profiling of scleroderma skin reveals robust signatures of disease that are imperfectly reflected in the transcript profiles of explanted fibroblasts. Arthritis Rheum. 54, 1961–1973 (2006).
Whitfield, M. L. et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc. Natl Acad. Sci. USA 100, 12319–12324 (2003).
Milano, A. et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE 3, e2696 (2008).
Tan, F. K. et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford) 45, 694–702 (2006).
Hsu, E. et al. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum. 63, 783–794 (2011).
Leroy, E. C. Connective tissue synthesis by scleroderma skin fibroblasts in cell culture. J. Exp. Med. 135, 1351–1362 (1972).
LeRoy, E. C. Increased collagen synthesis by scleroderma skin fibroblasts in vitro: a possible defect in the regulation or activation of the scleroderma fibroblast. J. Clin. Invest. 54, 880–889 (1974).
Ihn, H. Autocrine TGF-β signaling in the pathogenesis of systemic sclerosis. J. Dermatol. Sci. 49, 103–113 (2008).
Ghosh, A. K., Mori, Y., Dowling, E. & Varga, J. Trichostatin A blocks TGF-β-induced collagen gene expression in skin fibroblasts: involvement of Sp1. Biochem. Biophys. Res. Commun. 354, 420–426 (2007).
Bechtel, W. et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 16, 544–550 (2010).
Wang, Y., Fan, P. S. & Kahaleh, B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 54, 2271–2279 (2006).
Pandit, K. V., Milosevic, J. & Kaminski, N. MicroRNAs in idiopathic pulmonary fibrosis. Transl. Res. 157, 191–199 (2011).
Pandit, K. V. et al. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 182, 220–229 (2011).
van Rooij, E. et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl Acad. Sci. USA 105, 13027–13032 (2008).
Maurer, B. et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 62, 1733–1743 (2010).
Dong, C. et al. Deficient Smad7 expression: a putative molecular defect in scleroderma. Proc. Natl Acad. Sci. USA 99, 3908–3913 (2002).
Coward, W. R., Watts, K., Feghali-Bostwick, C. A., Knox, A. & Pang, L. Defective histone acetylation is responsible for the diminished expression of cyclooxygenase 2 in idiopathic pulmonary fibrosis. Mol. Cell. Biol. 29, 4325–4339 (2009).
Hemmatazad, H. et al. Histone deacetylase 7, a potential target for the antifibrotic treatment of systemic sclerosis. Arthritis Rheum. 60, 1519–1529 (2009).
Kaimori, A. et al. Histone deacetylase inhibition suppresses the transforming growth factor β1-induced epithelial-to-mesenchymal transition in hepatocytes. Hepatology 52, 1033–1045 (2010).
Guo, W., Shan, B., Klingsberg, R. C., Qin, X. & Lasky, J. A. Abrogation of TGF-β1-induced fibroblast–myofibroblast differentiation by histone deacetylase inhibition. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L864–L870 (2009).
Rajkumar, V. S. et al. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res. Ther. 7, R1113–R1123 (2005).
Zhou, G. et al. Hypoxia-induced alveolar epithelial–mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L1120–L1130 (2009).
Li, Z. & Jimenez, S. A. Protein kinase Cδ and the c-Abl kinase are required for transforming growth factor β induction of endothelial–mesenchymal transition in vitro. Arthritis Rheum. 63, 2473–2483 (2011).
Tan, X., Dagher, H., Hutton, C. A. & Bourke, J. E. Effects of PPARγ ligands on TGF-β1-induced epithelial–mesenchymal transition in alveolar epithelial cells. Respir. Res. 11, 21 (2010).
Reka, A. K. et al. Peroxisome proliferator-activated receptor-γ activation inhibits tumor metastasis by antagonizing Smad3-mediated epithelial-mesenchymal transition. Mol. Cancer Ther. 9, 3221–3232 (2010).
Du, F. et al. Expression of snail in epidermal keratinocytes promotes cutaneous inflammation and hyperplasia conducive to tumor formation. Cancer Res. 70, 10080–10089 (2010).
Dees, C. et al. Inhibition of Notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum. 63, 1396–1404 (2011).
Kavian, N. et al. Targeting ADAM-17/notch signaling abrogates the development of systemic sclerosis in a murine model. Arthritis Rheum. 62, 3477–3487 (2010).
Greenbaum, L. E. Hedgehog signaling in biliary fibrosis. J. Clin. Invest. 118, 3263–3265 (2008).
Beutler, B. A. TLRs and innate immunity. Blood 113, 1399–1407 (2009).
Kim, D. et al. Induction of interferon-α by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-α activity with lung fibrosis. Arthritis Rheum. 58, 2163–2173 (2008).
York, M. R. et al. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and Toll-like receptor agonists. Arthritis Rheum. 56, 1010–1020 (2007).
Duan, H. et al. Combined analysis of monocyte and lymphocyte messenger RNA expression with serum protein profiles in patients with scleroderma. Arthritis Rheum. 58, 1465–1474 (2008).
Assassi, S. et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. 62, 589–598 (2010).
Eloranta, M. L. et al. Type I interferon system activation and association with disease manifestations in systemic sclerosis. Ann. Rheum. Dis. 69, 1396–1402 (2010).
Dieude, P. et al. Association between the IRF5 rs2004640 functional polymorphism and systemic sclerosis: a new perspective for pulmonary fibrosis. Arthritis Rheum. 60, 225–233 (2009).
Agarwal, S. K. et al. Toll-like receptor 3 upregulation by type I interferon in healthy and scleroderma dermal fibroblasts. Arthritis Res. Ther. 13, R3 (2011).
Farina, G. A. et al. Poly(I:C) drives type I IFN- and TGFβ-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J. Invest. Dermatol. 130, 2583–2593 (2010).
Hyde, D. M. & Giri, S. N. Polyinosinic-polycytidylic acid, an interferon inducer, ameliorates bleomycin-induced lung fibrosis in mice. Exp. Lung Res. 16, 533–546 (1990).
Miyake, K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin. Immunol. 19, 3–10 (2007).
Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 200, 500–503 (2003).
Arslan, F. et al. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ. Res. 108, 582–592 (2011).
Muro, A. F. et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 177, 638–645 (2008).
Seki, E. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 (2007).
Fineschi, S. et al. Antifibroblast antibodies in systemic sclerosis induce fibroblasts to produce profibrotic chemokines, with partial exploitation of toll-like receptor 4. Arthritis Rheum. 58, 3913–3923 (2008).
Csak, T. et al. Deficiency in myeloid differentiation factor-2 and Toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G433–G441 (2011).
Pulskens, W. P. et al. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J. Am. Soc. Nephrol. 21, 1299–1308 (2010).
Campbell, M. T. et al. Toll-like receptor 4: a novel signaling pathway during renal fibrogenesis. J. Surg. Res. 168, e61–e69 (2009).
He, Z., Zhu, Y. & Jiang, H. Inhibiting Toll-like receptor 4 signaling ameliorates pulmonary fibrosis during acute lung injury induced by lipopolysaccharide: an experimental study. Respir. Res. 10, 126 (2009).
Lafyatis, R. & York, M. Innate immunity and inflammation in systemic sclerosis. Curr. Opin. Rheumatol. 21, 617–622 (2009).
Del Galdo, F., Wermuth, P. J., Addya, S., Fortina, P. & Jimenez, S. A. NFκB activation and stimulation of chemokine production in normal human macrophages by the gadolinium-based magnetic resonance contrast agent Omniscan: possible role in the pathogenesis of nephrogenic systemic fibrosis. Ann. Rheum. Dis. 69, 2024–2033 (2010).
Asano, Y., Ihn, H., Yamane, K., Kubo, M. & Tamaki, K. Increased expression levels of integrin αvβ5 on scleroderma fibroblasts. Am. J. Pathol. 164, 1275–1292 (2004).
Asano, Y. et al. Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J. Immunol. 175, 7708–7718 (2005).
Horan, G. S. et al. Partial inhibition of integrin αvβ6 prevents pulmonary fibrosis without exacerbating inflammation. Am. J. Respir. Crit. Care Med. 177, 56–65 (2008).
Varga, J. & Pasche, B. Transforming growth factor β as a therapeutic target in systemic sclerosis. Nat. Rev. Rheumatol. 5, 200–206 (2009).
Sargent, J. L. et al. A TGFβ-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J. Invest. Dermatol. 130, 694–705 (2010).
Kawakami, T. et al. Increased expression of TGF-β receptors by scleroderma fibroblasts: evidence for contribution of autocrine TGF-β signaling to scleroderma phenotype. J. Invest. Dermatol. 110, 47–51 (1998).
Pannu, J., Nakerakanti, S., Smith, E., ten Dijke, P. & Trojanowska, M. Transforming growth factor-β receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways. J. Biol. Chem. 282, 10405–10413 (2007).
Pannu, J., Gardner, H., Shearstone, J. R., Smith, E. & Trojanowska, M. Increased levels of transforming growth factor β receptor type I and up-regulation of matrix gene program: a model of scleroderma. Arthritis Rheum. 54, 3011–3021 (2006).
Ihn, H. Scleroderma, fibroblasts, signaling, and excessive extracellular matrix. Curr. Rheumatol. Rep. 7, 156–162 (2005).
Ihn, H., Yamane, K., Kubo, M. & Tamaki, K. Blockade of endogenous transforming growth factor β signaling prevents up-regulated collagen synthesis in scleroderma fibroblasts: association with increased expression of transforming growth factor β receptors. Arthritis Rheum. 44, 474–480 (2001).
Chen, Y. et al. Heparan sulfate-dependent ERK activation contributes to the overexpression of fibrotic proteins and enhanced contraction by scleroderma fibroblasts. Arthritis Rheum. 58, 577–585 (2008).
Jun, J. B. et al. Scleroderma fibroblasts demonstrate enhanced activation of Akt (protein kinase B) in situ. J. Invest. Dermatol. 124, 298–303 (2005).
Pannu, J. et al. Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum. 58, 2528–2537 (2008).
Bhattacharyya, S. et al. Smad-independent transforming growth factor-β regulation of early growth response-1 and sustained expression in fibrosis: implications for scleroderma. Am. J. Pathol. 173, 1085–1099 (2008).
Chen, S. J. et al. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-β: involvement of Smad 3. J. Invest. Dermatol. 112, 49–57 (1999).
Ghosh, A. K., Yuan, W., Mori, Y. & Varga, J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-β involves functional cooperation with p300/CBP transcriptional coactivators. Oncogene 19, 3546–3555 (2000).
Hong, M. et al. Non-Smad TGF-β signaling regulated by focal adhesion kinase binding the P85 subunit of phosphatidylinositol 3-kinase. J. Biol. Chem. 286, 17841–17850 (2011).
Shi-wen, X. et al. Requirement of transforming growth factor β-activated kinase 1 for transforming growth factor β-induced α-smooth muscle actin expression and extracellular matrix contraction in fibroblasts. Arthritis Rheum. 60, 234–241 (2009).
Varga, J. Scleroderma and Smads: dysfunctional Smad family dynamics culminating in fibrosis. Arthritis Rheum. 46, 1703–1713 (2002).
Ihn, H., Yamane, K., Asano, Y., Jinnin, M. & Tamaki, K. Constitutively phosphorylated Smad3 interacts with Sp1 and p300 in scleroderma fibroblasts. Rheumatology (Oxford) 45, 157–165 (2006).
Asano, Y., Ihn, H., Yamane, K., Kubo, M. & Tamaki, K. Impaired Smad7–Smurf-mediated negative regulation of TGF-β signaling in scleroderma fibroblasts. J. Clin. Invest. 113, 253–264 (2004).
Mori, Y., Chen, S. J. & Varga, J. Expression and regulation of intracellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 48, 1964–1978 (2003).
Bhattacharyya, S. et al. Fibroblast expression of the coactivator p300 governs the intensity of profibrotic response to transforming growth factor β. Arthritis Rheum. 52, 1248–1258 (2005).
Daniels, C. E. et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-β and prevents bleomycin-mediated lung fibrosis. J. Clin. Invest. 114, 1308–1316 (2004).
Bhattacharyya, S. et al. A non-Smad mechanism of fibroblast activation by transforming growth factor-β via c-Abl and EGR-1: selective modulation by imatinib mesylate. Oncogene 28, 1285–1297 (2009).
Rajkumar, V. S. et al. Platelet-derived growth factor-β receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am. J. Pathol. 169, 2254–2265 (2006).
Distler, J. H. et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 56, 311–322 (2007).
Vittal, R. et al. Effects of the protein kinase inhibitor, imatinib mesylate, on epithelial/mesenchymal phenotypes: implications for treatment of fibrotic diseases. J. Pharmacol. Exp. Ther. 321, 35–44 (2007).
Daniels, C. E. et al. Imatinib treatment for idiopathic pulmonary fibrosis: randomized placebo-controlled trial results. Am. J. Respir. Crit. Care Med. 181, 604–610 (2010).
Bhattacharyya, S. et al. Early growth response transcription factors: key mediators of fibrosis and novel targets for anti-fibrotic therapy. Matrix Biol. 30, 235–242 (2011).
Wu, M. et al. Essential roles for early growth response transcription factor EGR-1 in tissue fibrosis and wound healing. Am. J. Pathol. 175, 1041–1055 (2009).
Chen, S. J. et al. The early-immediate gene EGR-1 is induced by transforming growth factor-β and mediates stimulation of collagen gene expression. J. Biol. Chem. 281, 21183–21197 (2006).
Bhattacharyya, S. et al. The transcriptional cofactor Nab2 is induced by TGF-β and suppresses fibroblast activation: physiological roles and impaired expression in scleroderma. PLoS ONE 4, e7620 (2009).
Bhattacharyya, S. et al. EGR-1 induces a profibrotic injury/repair gene program associated with systemic sclerosis. PLoS ONE 6, e2 3082.
Leask, A. Towards an anti-fibrotic therapy for scleroderma: targeting myofibroblast differentiation and recruitment. Fibrogenesis Tissue Repair 3, 8 (2010).
Sonnylal, S. et al. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 62, 1523–1532 (2010).
Liu, S., Shi-wen, X., Abraham, D. J. & Leask, A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 63, 239–246 (2011).
Fonseca, C. et al. A polymorphism in the CTGF promoter region associated with systemic sclerosis. N. Engl. J. Med. 357, 1210–1220 (2007).
Xu, S. W. et al. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J. Biol. Chem. 279, 23098–23103 (2004).
Abraham, D. J. et al. Increased levels of endothelin-1 and differential endothelin type A and B receptor expression in scleroderma-associated fibrotic lung disease. Am. J. Pathol. 151, 831–841 (1997).
Shi-wen, X. et al. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor β in human lung fibroblasts. Arthritis Rheum. 56, 4189–4194 (2007).
Schroll, S. et al. Improvement of bleomycin-induced pulmonary hypertension and pulmonary fibrosis by the endothelin receptor antagonist Bosentan. Respir. Physiol. Neurobiol. 170, 32–36 (2010).
King, T. E. Jr et al. BUILD-3: A randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 184, 92–99 (2011).
Moon, R. T., Kohn, A. D., De Ferrari, G. V. & Kaykas, A. WNT and β-catenin signalling: diseases and therapies. Nat. Rev. Genet. 5, 691–701 (2004).
Vlad, A., Röhrs, S., Klein-Hitpass, L. & Müller, O. The first five years of the Wnt targetome. Cell Signal. 20, 795–802 (2008).
Carthy, J. M., Garmaroudi, F. S., Luo, Z. & McManus, B. M. Wnt3a induces myofibroblast differentiation by upregulating TGF-β signaling through SMAD2 in a β-catenin-dependent manner. PLoS ONE 6, e19809.
Chang, W. et al. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of β-catenin. J. Biol. Chem. 285, 8196–8206 (2010).
Thorne, C. A. et al. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat. Chem. Biol. 6, 829–836 (2010).
Wei, J. et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 63, 1707–1717 (2011).
Konigshoff, M. & Eickelberg, O. WNT signaling in lung disease: a failure or a regeneration signal? Am. J. Respir. Cell Mol. Biol. 42, 21–31 (2010).
Bayle, J. et al. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J. Invest. Dermatol. 128, 871–881 (2008).
Selman, M., Pardo, A. & Kaminski, N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 5, e62 (2008).
Chilosi, M. et al. Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am. J. Pathol. 162, 1495–1502 (2003).
Konigshoff, M. et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J. Clin. Invest. 119, 772–787 (2009).
Lam, A. P. et al. Nuclear β-catenin is increased in SSc pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am. J. Respir. Cell Mol. Biol. http://dx.doi.org/10.1165/rcmb.2010-0113OC.
Bielefeld, K. A. et al. Fibronectin and β-catenin act in a regulatory loop in dermal fibroblasts to modulate cutaneous healing. J. Biol. Chem. 286, 27687–27697 (2011).
He, W., Kang, Y. S., Dai, C. & Liu, Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 22, 90–103 (2011).
Henderson, W. R., Jr et al. Inhibition of Wnt/β-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl Acad. Sci. USA 107, 14309–14314 (2010).
Hinz, B. & Gabbiani, G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol. Rep. 2, 78 (2010).
Georges, P. C. et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G1147–G1154 (2007).
Wipff, P. J., Rifkin, D. B., Meister, J. J. & Hinz, B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell Biol. 179, 1311–1323 (2007).
Liu, F. et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 190, 693–706 (2010).
Margadant, C. & Sonnenberg, A. Integrin–TGF-β crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11, 97–105 (2010).
Eckes, B. et al. Fibroblast-matrix interactions in wound healing and fibrosis. Matrix Biol. 19, 325–332 (2000).
Liu, S., Kapoor, M., Denton, C. P., Abraham, D. J. & Leask, A. Loss of β1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model. Arthritis Rheum. 60, 2817–2821 (2009).
Liu, S. et al. Expression of integrin β1 by fibroblasts is required for tissue repair in vivo. J. Cell Sci. 123, 3674–3682 (2010).
Munger, J. S. et al. The integrin αvβ6 binds and activates latent TGF β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96, 319–328 (1999).
Worthington, J. J., Klementowicz, J. E. & Travis, M. A. TGFβ: a sleeping giant awoken by integrins. Trends Biochem. Sci. 36, 47–54 (2011).
Hakkinen, L. et al. Increased expression of β6-integrin in skin leads to spontaneous development of chronic wounds. Am. J. Pathol. 164, 229–242 (2004).
Asano, Y., Ihn, H., Yamane, K., Jinnin, M. & Tamaki, K. Increased expression of integrin αvβ5 induces the myofibroblastic differentiation of dermal fibroblasts. Am. J. Pathol. 168, 499–510 (2006).
Loeys, B. L. et al. Mutations in fibrillin-1 cause congenital scleroderma: stiff skin syndrome. Sci. Transl. Med. 2, 23–20 (2010).
Henault, J., Robitaille, G., Senecal, J. L. & Raymond, Y. DNA topoisomerase I binding to fibroblasts induces monocyte adhesion and activation in the presence of anti-topoisomerase I autoantibodies from systemic sclerosis patients. Arthritis Rheum. 54, 963–973 (2006).
Chizzolini, C., Brembilla, N. C., Montanari, E. & Truchetet, M. E. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun. Rev. 10, 276–281 (2011).
Zhou, X. et al. Autoantibodies to fibrillin-1 activate normal human fibroblasts in culture through the TGF-β pathway to recapitulate the “scleroderma phenotype”. J. Immunol. 175, 4555–4560 (2005).
Brinckmann, J. et al. Absence of autoantibodies against correctly folded recombinant fibrillin-1 protein in systemic sclerosis patients. Arthritis Res. Ther. 7, R1221–R1226 (2005).
Baroni, S. S. et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N. Engl. J. Med. 354, 2667–2676 (2006).
Classen, J. F. et al. Lack of evidence of stimulatory autoantibodies to platelet-derived growth factor receptor in patients with systemic sclerosis. Arthritis Rheum. 60, 1137–1144 (2009).
Loizos, N. et al. Lack of detection of agonist activity by antibodies to platelet-derived growth factor receptor α in a subset of normal and systemic sclerosis patient sera. Arthritis Rheum. 60, 1145–1151 (2009).
Distler, J. H. et al. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 56, 4203–4215 (2007).
Bogatkevich, G. S. et al. Antiinflammatory and antifibrotic effects of the oral direct thrombin inhibitor dabigatran etexilate in a murine model of interstitial lung disease. Arthritis Rheum. 63, 1416–1425 (2011).
Olson, L. E. & Soriano, P. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev. Cell 16, 303–313 (2009).
Dees, C. et al. Platelet-derived serotonin links vascular disease and tissue fibrosis. J. Exp. Med. 208, 961–972 (2011).
Gabrielli, A., Avvedimento, E. V. & Krieg, T. Scleroderma. N. Engl. J. Med. 360, 1989–2003 (2009).
Hecker, L. et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 15, 1077–1081 (2009).
Servettaz, A. et al. Radical oxygen species production induced by advanced oxidation protein products predicts clinical evolution and response to treatment in systemic sclerosis. Ann. Rheum. Dis. 66, 1202–1209 (2007).
Huang, S. K. & Peters-Golden, M. Eicosanoid lipid mediators in fibrotic lung diseases: ready for prime time? Chest 133, 1442–1450 (2008).
Oga, T. et al. Prostaglandin F2α receptor signaling facilitates bleomycin-induced pulmonary fibrosis independently of transforming growth factor-β. Nat. Med. 15, 1426–1430 (2009).
Rancoule, C. et al. Lysophosphatidic acid-1-receptor targeting agents for fibrosis. Expert Opin. Investig. Drugs 20, 657–667 (2011).
Tager, A. M. et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med. 14, 45–54 (2008).
Castelino, F. V. et al. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 63, 1405–1415 (2011).
Xu, M. Y. et al. Lysophosphatidic acid induces αvβ6 integrin-mediated TGF-β activation via the LPA2 receptor and the small G protein Gαq . Am. J. Pathol. 174, 1264–1279 (2009).
Wei, J., Bhattacharyya, S. & Varga, J. Peroxisome proliferator-activated receptor γ: innate protection from excessive fibrogenesis and potential therapeutic target in systemic sclerosis. Curr. Opin. Rheumatol. 22, 671–676 (2010).
Ghosh, A. K. et al. Peroxisome proliferator-activated receptor-γ abrogates Smad-dependent collagen stimulation by targeting the p300 transcriptional coactivator. FASEB J. 23, 2968–2977 (2009).
Ghosh, A. K. et al. Disruption of transforming growth factor β signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor γ. Arthritis Rheum. 50, 1305–1318 (2004).
Kapoor, M. et al. Loss of peroxisome proliferator-activated receptor γ in mouse fibroblasts results in increased susceptibility to bleomycin-induced skin fibrosis. Arthritis Rheum. 60, 2822–2829 (2009).
Karnik, P. et al. Hair follicle stem cell-specific PPARγ deletion causes scarring alopecia. J. Invest. Dermatol. 129, 1243–1257 (2009).
Wu, M. et al. Rosiglitazone abrogates bleomycin-induced scleroderma and blocks profibrotic responses through peroxisome proliferator-activated receptor-γ. Am. J. Pathol. 174, 519–533 (2009).
Zheng, F. et al. Upregulation of type I collagen by TGF-β in mesangial cells is blocked by PPARγ activation. Am. J. Physiol. Renal Physiol. 282, F639–F648 (2002).
Miyahara, T. et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J. Biol. Chem. 275, 35715–35722 (2000).
Culver, D. A. et al. Peroxisome proliferator-activated receptor γ activity is deficient in alveolar macrophages in pulmonary sarcoidosis. Am. J. Respir. Cell Mol. Biol. 30, 1–5 (2004).
Wei, J. et al. PPARγ downregulation by TGFβ in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS ONE 5, e13778 (2010).
Fang, F. et al. The early growth response gene EGR2 (alias Krox20) is a novel transcriptional target of transforming growth factor-β that is up-regulated in systemic sclerosis and mediates profibrotic responses. Am. J. Pathol. 178, 2077–2090 (2011).
Kubo, M. et al. Persistent down-regulation of Fli1, a suppressor of collagen transcription, in fibrotic scleroderma skin. Am. J. Pathol. 163, 571–581 (2003).
Jinnin, M., Ihn, H., Mimura, Y., Asano, Y. & Tamaki, K. Involvement of the constitutive complex formation of c-Ski/SnoN with Smads in the impaired negative feedback regulation of transforming growth factor β signaling in scleroderma fibroblasts. Arthritis Rheum. 56, 1694–1705 (2007).
Bu, S. et al. Dihydrosphingosine 1-phosphate has a potent antifibrotic effect in scleroderma fibroblasts via normalization of phosphatase and tensin homolog levels. Arthritis Rheum. 62, 2117–2126 (2010).
Parapuram, S. K. et al. Loss of PTEN expression by dermal fibroblasts causes skin fibrosis. J. Invest. Dermatol. http://dx.doi.org/10.1038/jid.2011.156.
Acknowledgements
This work was partially supported by grants from the National Institutes of Health (AR 42309) and the Scleroderma Research Foundation. We are grateful to Carol Feghali-Bostwick, Warren Tourtellotte, Monique Hinchcliff, Robert Lafyatis, Michael Whitfield, Cara Gottardi, Carol Artlett, Maria Trojanowska and members of the Northwestern Scleroderma Program for valuable discussions.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching data, discussing content, writing, and review/editing of the manuscript before subscription.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bhattacharyya, S., Wei, J. & Varga, J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol 8, 42–54 (2012). https://doi.org/10.1038/nrrheum.2011.149
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2011.149
This article is cited by
-
Heavy-chain antibody targeting of CD38 NAD+ hydrolase ectoenzyme to prevent fibrosis in multiple organs
Scientific Reports (2023)
-
Unravelling morphoea aetiopathogenesis by next-generation sequencing of paired skin biopsies
Archives of Dermatological Research (2023)
-
Autologous hematopoietic stem cell transplantation promotes connective tissue remodeling in systemic sclerosis patients
Arthritis Research & Therapy (2022)
-
Dersimelagon, a novel oral melanocortin 1 receptor agonist, demonstrates disease-modifying effects in preclinical models of systemic sclerosis
Arthritis Research & Therapy (2022)
-
Centromere defects, chromosome instability, and cGAS-STING activation in systemic sclerosis
Nature Communications (2022)