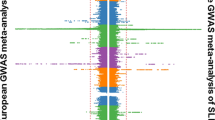
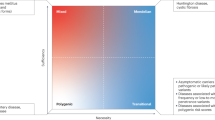
Abstract
Autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus are generally considered multifactorial—that is, they involve both genetic and environmental factors. Technical advances in human genetics over the past 5 years have enabled the survey of the entire human genome for disease susceptibility genes and have contributed to a greater understanding of the molecular mechanisms underlying autoimmunity. Among the genetic predisposition factors identified to date, some variants have been found to be restricted to specific ethnic groups, which might reflect migration history and the natural selection that shaped genetic variation in these populations. Other genetic factors could also have exerted different magnitudes of risk for the disease among the different populations, which might be explained by their interactions with other genetic and environmental factors. These pieces of evidence suggest that substantial heterogeneity exists in the genetics underlying autoimmunity among different ethnic populations. This Review discusses the genetic heterogeneity in autoimmunity, with a focus on rheumatoid arthritis, between Asian and European populations. In addition to the most-studied and well-characterized gene HLA-DRB1, we will also describe examples of the gene–environment interactions between PADI4 and smoking, and the gene–gene interactions between PTPN22 and FCRL3.
Key Points
-
Genome-wide association studies have revealed multiple genetic risk factors for rheumatoid arthritis (RA) in addition to the well-studied HLA-DRB1 locus
-
Some of the RA-associated genetic factors are restricted to specific ethnic populations, indicating the presence of genetic heterogeneity in RA
-
The genetic heterogeneity might be primarily explained by the exclusive presence of a disease susceptibility allele in a particular population (for example, PTPN22 variant in European populations and HLA-DRB1*0901 in Asian populations)
-
Gene–environment interactions and gene–gene interactions might affect the contribution of each genetic factor to disease susceptibility, and account for the genetic heterogeneity between the populations
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Plenge, R. M. Recent progress in rheumatoid arthritis genetics: one step towards improved patient care. Curr. Opin. Rheumatol. 21, 262–271 (2009).
Plenge, R. M. et al. TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N. Engl. J. Med. 357, 1199–1209 (2007).
Plenge, R. M. et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat. Genet. 39, 1477–1482 (2007).
Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007).
Gregersen, P. K. et al. REL, encoding a member of the NF-κB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat. Genet. 41, 820–823 (2009).
Shimane, K. et al. The association of a nonsynonymous single-nucleotide polymorphism in TNFAIP3 with systemic lupus erythematosus and rheumatoid arthritis in the Japanese population. Arthritis Rheum. 62, 574–579 (2010).
Remmers, E. F. et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N. Engl. J. Med. 357, 977–986 (2007).
Lee, H. S. et al. Association of STAT4 with rheumatoid arthritis in the Korean population. Mol. Med. 13, 455–460 (2007).
Kobayashi, S. et al. Association of STAT4 with susceptibility to rheumatoid arthritis and systemic lupus erythematosus in the Japanese population. Arthritis Rheum. 58, 1940–1946 (2008).
Abdel-Nasser, A. M., Rasker, J. J. & Valkenburg, H. A. Epidemiological and clinical aspects relating to the variability of rheumatoid arthritis. Semin. Arthritis Rheum. 27, 123–140 (1997).
Alamanos, Y. & Drosos, A. A. Epidemiology of adult rheumatoid arthritis. Autoimmun. Rev. 4, 130–136 (2005).
Ferucci, E. D., Templin, D. W. & Lanier, A. P. Rheumatoid arthritis in American Indians and Alaska Natives: a review of the literature. Semin. Arthritis Rheum. 34, 662–667 (2005).
Klareskog, L. et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 54, 38–46 (2006).
Gabriel, S. E. The epidemiology of rheumatoid arthritis. Rheum. Dis. Clin. North Am. 27, 269–281 (2001).
Kallberg, H. et al. Gene–gene and gene–environment interactions involving HLA-DRB1, PTPN22, and smoking in two subsets of rheumatoid arthritis. Am. J. Hum. Genet. 80, 867–875 (2007).
Lee, H. S. et al. Interaction between smoking, the shared epitope, and anti-cyclic citrullinated peptide: a mixed picture in three large North American rheumatoid arthritis cohorts. Arthritis Rheum. 56, 1745–1753 (2007).
Prugnolle, F. et al. Pathogen-driven selection and worldwide HLA class I diversity. Curr. Biol. 15, 1022–1027 (2005).
Verity, D. H., Marr, J. E., Ohno, S., Wallace, G. R. & Stanford, M. R. Behçet's disease, the Silk Road and HLA-B51: historical and geographical perspectives. Tissue Antigens 54, 213–220 (1999).
Newton, J. L., Harney, S. M., Wordsworth, B. P. & Brown, M. A. A review of the MHC genetics of rheumatoid arthritis. Genes Immun. 5, 151–157 (2004).
du Montcel, S. T. et al. New classification of HLA-DRB1 alleles supports the shared epitope hypothesis of rheumatoid arthritis susceptibility. Arthritis Rheum. 52, 1063–1068 (2005).
de Vries, N., Tijssen, H., van Riel, P. L. & van de Putte, L. B. Reshaping the shared epitope hypothesis: HLA-associated risk for rheumatoid arthritis is encoded by amino acid substitutions at positions 67–74 of the HLA-DRB1 molecule. Arthritis Rheum. 46, 921–928 (2002).
Mattey, D. L. et al. HLA-DRB1 alleles encoding an aspartic acid at position 70 protect against development of rheumatoid arthritis. J. Rheumatol. 28, 232–239 (2001).
Evans, T. I., Han, J., Singh, R. & Moxley, G. The genotypic distribution of shared-epitope DRB1 alleles suggests a recessive mode of inheritance of the rheumatoid arthritis disease-susceptibility gene. Arthritis Rheum. 38, 1754–1761 (1995).
Stastny, P. Mixed lymphocyte cultures in rheumatoid arthritis. J. Clin. Invest. 57, 1148–1157 (1976).
Lee, H. S. et al. Increased susceptibility to rheumatoid arthritis in Koreans heterozygous for HLA-DRB1*0405 and *0901. Arthritis Rheum. 50, 3468–3475 (2004).
Kochi, Y. et al. Analysis of single-nucleotide polymorphisms in Japanese rheumatoid arthritis patients shows additional susceptibility markers besides the classic shared epitope susceptibility sequences. Arthritis Rheum. 50, 63–71 (2004).
Kong, K. F., Yeap, S. S., Chow, S. K. & Phipps, M. E. HLA-DRB1 genes and susceptibility to rheumatoid arthritis in three ethnic groups from Malaysia. Autoimmunity 35, 235–239 (2002).
Wakitani, S. et al. The relationship between HLA-DRB1 alleles and disease subsets of rheumatoid arthritis in Japanese. Br. J. Rheumatol. 36, 630–636 (1997).
Chan, S. H., Lin, Y. N., Wee, G. B., Koh, W. H. & Boey, M. L. HLA class 2 genes in Singaporean Chinese rheumatoid arthritis. Br. J. Rheumatol. 33, 713–717 (1994).
Gregersen, P. K., Silver, J. & Winchester, R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 30, 1205–1213 (1987).
Wakitani, S. et al. The homozygote of HLA-DRB1*0901, not its heterozygote, is associated with rheumatoid arthritis in Japanese. Scand. J. Rheumatol. 27, 381–382 (1998).
Klareskog, L., Ronnelid, J., Lundberg, K., Padyukov, L. & Alfredsson, L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu. Rev. Immunol. 26, 651–675 (2008).
Auger, I. et al. Influence of HLA-DR genes on the production of rheumatoid arthritis-specific autoantibodies to citrullinated fibrinogen. Arthritis Rheum. 52, 3424–3432 (2005).
van Gaalen, F. A. et al. Association between HLA class II genes and autoantibodies to cyclic citrullinated peptides (CCPs) influences the severity of rheumatoid arthritis. Arthritis Rheum. 50, 2113–2121 (2004).
Furuya, T. et al. Differential association of HLA-DRB1 alleles in Japanese patients with early rheumatoid arthritis in relationship to autoantibodies to cyclic citrullinated peptide. Clin. Exp. Rheumatol. 25, 219–224 (2007).
Irigoyen, P. et al. Regulation of anti-cyclic citrullinated peptide antibodies in rheumatoid arthritis: contrasting effects of HLA-DR3 and the shared epitope alleles. Arthritis Rheum. 52, 3813–3818 (2005).
Verpoort, K. N. et al. Association of HLA-DR3 with anti-cyclic citrullinated peptide antibody-negative rheumatoid arthritis. Arthritis Rheum. 52, 3058–3062 (2005).
Suzuki, A. et al. Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nat. Genet. 34, 395–402 (2003).
Nakashima, K. et al. Molecular characterization of peptidylarginine deiminase in HL-60 cells induced by retinoic acid and 1α, 25-dihydroxyvitamin D3 . J. Biol. Chem. 274, 27786–27792 (1999).
Cha, S. et al. Association of anti-cyclic citrullinated peptide antibody levels with PADI4 haplotypes in early rheumatoid arthritis and with shared epitope alleles in very late rheumatoid arthritis. Arthritis Rheum. 56, 1454–1463 (2007).
Gandjbakhch, F. et al. A functional haplotype of PADI4 gene in rheumatoid arthritis: positive correlation in a French population. J. Rheumatol. 36, 881–886 (2009).
Ikari, K. et al. Association between PADI4 and rheumatoid arthritis: a replication study. Arthritis Rheum. 52, 3054–3057 (2005).
Kang, C. P. et al. A functional haplotype of the PADI4 gene associated with increased rheumatoid arthritis susceptibility in Koreans. Arthritis Rheum. 54, 90–96 (2006).
Plenge, R. M. et al. Replication of putative candidate-gene associations with rheumatoid arthritis in >4,000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4. Am. J. Hum. Genet. 77, 1044–1060 (2005).
Burr, M. L. et al. PADI4 genotype is not associated with rheumatoid arthritis in a large UK caucasian population. Ann. Rheum. Dis. doi:10.1136/ard.2009.111294.
Harney, S. M. et al. Genetic and genomic studies of PADI4 in rheumatoid arthritis. Rheumatology (Oxford) 44, 869–872 (2005).
Makrygiannakis, D. et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann. Rheum. Dis. 67, 1488–1492 (2008).
Mei, L. et al. Evaluating gene × gene and gene × smoking interaction in rheumatoid arthritis using candidate genes in GAW15. BMC Proc. 1 (Suppl. 1), S17 (2007).
Begovich, A. B. et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am. J. Hum. Genet. 75, 330–337 (2004).
Gregersen, P. K., Lee, H. S., Batliwalla, F. & Begovich, A. B. PTPN22: setting thresholds for autoimmunity. Semin. Immunol. 18, 214–223 (2006).
Mori, M., Yamada, R., Kobayashi, K., Kawaida, R. & Yamamoto, K. Ethnic differences in allele frequency of autoimmune-disease-associated SNPs. J. Hum. Genet. 50, 264–266 (2005).
Zhang, Z. H. et al. PTPN22 allele polymorphisms in 15 Chinese populations. Int. J. Immunogenet. 35, 433–437 (2008).
Cohen, S., Dadi, H., Shaoul, E., Sharfe, N. & Roifman, C. M. Cloning and characterization of a lymphoid-specific, inducible human protein tyrosine phosphatase, Lyp. Blood 93, 2013–2024 (1999).
Vang, T. et al. Protein tyrosine phosphatases in autoimmunity. Annu. Rev. Immunol. 26, 29–55 (2008).
Vang, T. et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat. Genet. 37, 1317–1319 (2005).
Arechiga, A. F. et al. Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J. Immunol. 182, 3343–3347 (2009).
Kochi, Y. et al. A functional variant in FCRL3, encoding Fc receptor-like 3, is associated with rheumatoid arthritis and several autoimmunities. Nat. Genet. 37, 478–485 (2005).
Ikari, K. et al. Supportive evidence for a genetic association of the FCRL3 promoter polymorphism with rheumatoid arthritis. Ann. Rheum. Dis. 65, 671–673 (2006).
Umemura, T. et al. Genetic association of Fc receptor-like 3 polymorphisms with autoimmune pancreatitis in Japanese patients. Gut 55, 1367–1368 (2006).
Shimada, M. et al. Association of autoimmune disease-related gene polymorphisms with chronic graft-versus-host disease. Br. J. Haematol. 139, 458–463 (2007).
Simmonds, M. J. et al. Contribution of single nucleotide polymorphisms within FCRL3 and MAP3K7IP2 to the pathogenesis of Graves' disease. J. Clin. Endocrinol. Metab. 91, 1056–1061 (2006).
Burton, P. R. et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Newman, W. G. et al. Rheumatoid arthritis association with the FCRL3 −169C polymorphism is restricted to PTPN22 1858T-homozygous individuals in a Canadian population. Arthritis Rheum. 54, 3820–3827 (2006).
Davis, R. S. Fc receptor-like molecules. Annu. Rev. Immunol. 25, 525–560 (2007).
Mendoza, J. L. et al. FcRL3 gene promoter variant is associated with peripheral arthritis in Crohn's disease. Inflamm. Bowel Dis. 15, 1351–1357 (2009).
Xu, M. J., Zhao, R., Cao, H. & Zhao, Z. J. SPAP2, an Ig family receptor containing both ITIMs and ITAMs. Biochem. Biophys. Res. Commun. 293, 1037–1046 (2002).
Kochi, Y. et al. FCRL3, an autoimmune susceptibility gene, has inhibitory potential on B-cell receptor-mediated signaling. J. Immunol. 183, 5502–5510 (2009).
Sakaguchi, N. et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426, 454–460 (2003).
Kumar, K. R. et al. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science 312, 1665–1669 (2006).
Acknowledgements
The authors thank members of the Laboratory for Autoimmune Diseases, RIKEN, Tokyo for their assistance. This work was supported by grants from the Center for Genomic Medicine, RIKEN; the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Leading Project); and the Ministry of Health, Labor, and Welfare of Japan (Research on Intractable Diseases).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kochi, Y., Suzuki, A., Yamada, R. et al. Ethnogenetic heterogeneity of rheumatoid arthritis—implications for pathogenesis. Nat Rev Rheumatol 6, 290–295 (2010). https://doi.org/10.1038/nrrheum.2010.23
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.23
This article is cited by
-
Isotypes of autoantibodies against novel differential 4-hydroxy-2-nonenal-modified peptide adducts in serum is associated with rheumatoid arthritis in Taiwanese women
BMC Medical Informatics and Decision Making (2021)
-
The 3′-UTR (CA)n microsatellite on CD40LG gene as a possible genetic marker for rheumatoid arthritis in Mexican population: impact on CD40LG mRNA expression
Clinical Rheumatology (2018)
-
Genomics, transcriptomics and proteomics to elucidate the pathogenesis of rheumatoid arthritis
Rheumatology International (2017)
-
A longitudinal genome-wide association study of anti-tumor necrosis factor response among Japanese patients with rheumatoid arthritis
Arthritis Research & Therapy (2016)
-
Decreased severity of experimental autoimmune arthritis in peptidylarginine deiminase type 4 knockout mice
BMC Musculoskeletal Disorders (2016)