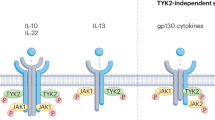
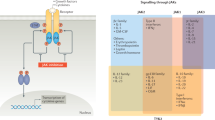
Abstract
As critical regulators of numerous cell signaling pathways, tyrosine kinases are implicated in the pathogenesis of several diseases, including rheumatoid arthritis (RA). In the absence of disease, synoviocytes produce factors that provide nutrition and lubrication for the surrounding cartilage tissue; few cellular infiltrates are seen in the synovium. In RA, however, macrophages, neutrophils, T cells and B cells infiltrate the synovium and produce cytokines, chemokines and degradative enzymes that promote inflammation and joint destruction. In addition, the synovial lining expands owing to the proliferation of synoviocytes and infiltration of inflammatory cells to form a pannus, which invades the surrounding bone and cartilage. Many of these cell responses are regulated by tyrosine kinases that operate in specific signaling pathways, and inhibition of a number of these kinases might be expected to provide benefit in RA.
Key Points
-
Rheumatoid arthritis (RA) is characterized by leukocyte infiltration, synoviocyte hyperplasia and osteoclastogenesis, and tyrosine kinases have key roles in the signaling pathways that regulate these processes
-
Inhibition of platelet-derived growth factor receptors, vascular endothelial growth factor receptors and TIE receptors might reduce synovial hyperplasia and angiogenesis
-
Inhibition of colony-stimulating factor receptor-1 and Src might reduce monocyte maturation and osteoclastogenesis
-
Blocking signaling through Bruton's tyrosine kinase might reduce B-cell and T-cell activation
-
Blocking KIT activation might induce mast cell apoptosis, thereby reducing the production of inflammatory cytokines and degradative molecules in the synovium
-
Imatinib, which inhibits several tyrosine kinases, and more-specific inhibitors of Janus kinases and Syk, have already shown efficacy in the treatment of RA; however, toxicity remains an issue
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Firestein, G. S. Evolving concepts of rheumatoid arthritis. Nature 423, 356–361 (2003).
Sano, H. et al. Coexpression of phosphotyrosine-containing proteins, platelet-derived growth factor-B, and fibroblast growth factor-1 in situ in synovial tissues of patients with rheumatoid arthritis and Lewis rats with adjuvant or streptococcal cell wall arthritis. J. Clin. Invest. 91, 553–565 (1993).
Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 103, 211–225 (2000).
Benati, D. & Baldari, C. T. SRC family kinases as potential therapeutic targets for malignancies and immunological disorders. Curr. Med. Chem. 15, 1154–1165 (2008).
Schindler, T. et al. Structural mechanism for STI-571 inhibition of Abelson tyrosine kinase. Science 289, 1938–1942 (2000).
Ikeda, M., Hosoda, Y., Hirose, S., Okada, Y. & Ikeda, E. Expression of vascular endothelial growth factor isoforms and their receptors Flt-1, KDR, and neuropilin-1 in synovial tissues of rheumatoid arthritis. J. Pathol. 191, 426–433 (2000).
Betsholtz, C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 15, 215–228 (2004).
Pohlers, D. et al. Expression of platelet-derived growth factors C and D in the synovial membrane of patients with rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 54, 788–794 (2006).
Watanabe, N. et al. Gene expression profile analysis of rheumatoid synovial fibroblast cultures revealing the overexpression of genes responsible for tumor-like growth of rheumatoid synovium. Biochem. Biophys. Res. Commun. 294, 1121–1129 (2002).
Reuterdahl, C. et al. Characterization of platelet-derived growth factor beta-receptor expressing cells in the vasculature of human rheumatoid synovium. Lab. Invest. 64, 321–329 (1991).
Paniagua, R. T. et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J. Clin. Invest. 116, 2633–2642 (2006).
Ando, W. et al. Imatinib mesylate inhibits osteoclastogenesis and joint destruction in rats with collagen-induced arthritis (CIA). J. Bone Miner. Metab. 24, 274–282 (2006).
Koyama, K. et al. Imatinib mesylate both prevents and treats the arthritis induced by type II collagen antibody in mice. Mod. Rheumatol. 17, 306–310 (2007).
Sandler, C. et al. Imatinib mesylate inhibits platelet derived growth factor stimulated proliferation of rheumatoid synovial fibroblasts. Biochem. Biophys. Res. Commun. 347, 31–35 (2006).
Kameda, H. et al. Imatinib mesylate inhibits proliferation of rheumatoid synovial fibroblast-like cells and phosphorylation of Gab adapter proteins activated by platelet-derived growth factor. Clin. Exp. Immunol. 144, 335–341 (2006).
Giatromanolaki, A. et al. The angiogenic pathway “vascular endothelial growth factor/flk-1(KDR)-receptor” in rheumatoid arthritis and osteoarthritis. J. Pathol. 194, 101–108 (2001).
Roy, H., Bhardwaj, S. & Ylä- Herttuala, S. Biology of vascular endothelial growth factors. FEBS Lett. 580, 2879–2887 (2006).
Ballara, S. et al. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis Rheum. 44, 2055–2064 (2001).
Sone, H. et al. Elevated levels of vascular endothelial growth factor in the sera of patients with rheumatoid arthritis correlation with disease activity. Life Sci. 69, 1861–1869 (2001).
Olszewski, W. L. et al. Lymph draining from foot joints in rheumatoid arthritis provides insight into local cytokine and chemokine production and transport to lymph nodes. Arthritis Rheum. 44, 541–549 (2001).
Tanaka, S. Signaling axis in osteoclast biology and therapeutic targeting in the RANKL/RANK/OPG system. Am. J. Nephrol. 27, 466–478 (2007).
Kim, W. U. et al. Interaction of vascular endothelial growth factor 165 with neuropilin-1 protects rheumatoid synoviocytes from apoptotic death by regulating Bcl-2 expression and Bax translocation. J. Immunol. 177, 5727–5735 (2006).
Paavonen, K. et al. Vascular endothelial growth factors C and D and their VEGFR-2 and 3 receptors in blood and lymphatic vessels in healthy and arthritic synovium. J. Rheumatol. 29, 39–45 (2002).
Fava, R. A. et al. Vascular permeability factor/endothelial growth factor (VPF/VEGF): accumulation and expression in human synovial fluids and rheumatoid synovial tissue. J. Exp. Med. 180, 341–346 (1994).
Pufe, T. et al. Splice variants VEGF121 and VEGF165 of the angiogenic peptide vascular endothelial cell growth factor are expressed in the synovial tissue of patients with rheumatoid arthritis. J. Rheumatol. 28, 1482–1485 (2001).
De Bandt, M. et al. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII, suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J. Immunol. 171, 4853–4859 (2003).
Lu, J. et al. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J. Immunol. 164, 5922–5927 (2000).
Jin, P. et al. Novel splice variants derived from the receptor tyrosine kinase superfamily are potential therapeutics for rheumatoid arthritis. Arthritis Res. Ther. 10, R73 (2008).
Murakami, M. et al. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood 108, 1849–1856 (2006).
Faivre, S. et al. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 6, 734–745 (2007).
Makinde, T. & Agrawal, D. K. Intra and extravascular transmembrane signalling of angiopoietin-1-Tie2 receptor in health and disease. J. Cell. Mol. Med. 12, 810–828 (2008).
Shahrara, S. et al. Differential expression of the angiogenic Tie receptor family in arthritic and normal synovial tissue. Arthritis Res. 4, 201–208 (2002).
Uchida, T. et al. Immunohistochemical localisation of protein tyrosine kinase receptors Tie-1 and Tie-2 in synovial tissue of rheumatoid arthritis: correlation with angiogenesis and synovial proliferation. Ann. Rheum. Dis. 59, 607–614 (2000).
Chen, Y., Donnelly, E., Kobayashi, H., Debusk, L. M. & Lin, P. C. Gene therapy targeting the Tie2 function ameliorates collagen-induced arthritis and protects against bone destruction. Arthritis Rheum. 52, 1585–1594 (2005).
Orozco, G. et al. Genetic basis of rheumatoid arthritis. Biomed. Pharmacother. 60, 656–662 (2006).
Yanaba, K. et al. B-lymphocyte contributions to human autoimmune disease. Immunol. Rev. 223, 284–299 (2008).
Jiang, A. et al. Different protein tyrosine kinases are required for B cell antigen receptor-mediated activation of extracellular signal-regulated kinase, c-Jun NH2-terminal kinase 1, and p38 mitogen-activated protein kinase. J. Exp. Med. 188, 1297–1306 (1998).
Blake, S. et al. The Src/ABL kinase inhibitor dasatinib (BMS-354825) inhibits function of normal human T-lymphocytes in vitro. Clin. Immunol. 127, 330–339 (2008).
Rokosz, L. L. et al. Kinase inhibitors as drugs for chronic inflammatory and immunological diseases: progress and challenges. Expert Opin. Ther. Targets 12, 883–903 (2008).
Felices, M. et al. Tec kinases in T cell and mast cell signaling. Adv. Immunol. 93, 145–184 (2007).
Pan, Z. et al. Discovery of selective irreversible inhibitors for Bruton's tyrosine kinase. ChemMedChem 2, 58–61 (2007).
Hantschel, O. et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc. Natl Acad. Sci. USA 104, 13283–13288 (2007).
Eklund, K. K. Mast cells in the pathogenesis of rheumatic diseases and as potential targets for anti-rheumatic therapy. Immunol. Rev. 217, 38–52 (2007).
Bakharevski, O. & Ryan, P. F. Mast cells as a target in the treatment of rheumatoid arthritis. Inflammopharmacology 7, 351–362 (1999).
Lee, D. M. et al. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science 297, 1689–1692 (2002).
Juurikivi, A. et al. Inhibition of c-kit tyrosine kinase by imatinib mesylate induces apoptosis in mast cells in rheumatoid synovia: a potential approach to the treatment of arthritis. Ann. Rheum. Dis. 64, 1126–1131 (2005).
Carsons, S. E. et al. Detection and quantitation of stem cell factor (kit ligand) in the synovial fluid of patients with rheumatic disease. J. Rheumatol. 27, 2798–2800 (2000).
Ceponis, A. et al. Expression of stem cell factor (SCF) and SCF receptor (c-kit) in synovial membrane in arthritis: correlation with synovial mast cell hyperplasia and inflammation. J. Rheumatol. 25, 2304–2314 (1998).
Olsson, N. et al. Demonstration of mast cell chemotactic activity in synovial fluid from rheumatoid patients. Ann. Rheum. Dis. 60, 187–193 (2001).
Adamopoulos, I. E. et al. Synovial fluid macrophages are capable of osteoclast formation and resorption. J. Pathol. 208, 35–43 (2006).
Takasugi, K. et al. Induction of tumour necrosis factor receptor-expressing macrophages by interleukin-10 and macrophage colony-stimulating factor in rheumatoid arthritis. Arthritis Res. Ther. 8, R126 (2006).
Nakano, K. et al. Rheumatoid synovial endothelial cells produce macrophage colony-stimulating factor leading to osteoclastogenesis in rheumatoid arthritis. Rheumatology (Oxford) 46, 597–603 (2007).
Seitz, M. et al. Constitutive mRNA and protein production of macrophage colony-stimulating factor but not of other cytokines by synovial fibroblasts from rheumatoid arthritis and osteoarthritis patients. Br. J. Rheumatol. 33, 613–619 (1994).
Campbell, I. K. et al. The colony-stimulating factors and collagen-induced arthritis: exacerbation of disease by M-CSF and G-CSF and requirement for endogenous M-CSF. J. Leukoc. Biol. 68, 144–150 (2000).
Bischof, R. J. et al. Exacerbation of acute inflammatory arthritis by the colony-stimulating factors CSF-1 and granulocyte macrophage (GM)-CSF: evidence of macrophage infiltration and local proliferation. Clin. Exp. Immunol. 119, 361–367 (2000).
Abd, A. H. et al. The role of macrophages in experimental arthritis induced by Streptococcus agalactiae sonicate: actions of macrophage colony-stimulating factor (CSF-1) and other macrophage-modulating agents. Lymphokine Cytokine Res. 10, 43–50 (1991).
Yang, Y. H. & Hamilton, J. A. Dependence of interleukin-1-induced arthritis on granulocyte-macrophage colony-stimulating factor. Arthritis Rheum. 44, 111–119 (2001).
Kitaura, H. et al. M-CSF mediates TNF-induced inflammatory osteolysis. J. Clin. Invest. 115, 3418–3427 (2005).
Conway, J. G. et al. Effects of the cFMS kinase inhibitor 5-(3-methoxy-4-[{4-methoxybenzyl}oxy]benzyl)pyrimidine-2, 4-diamine (GW2580) in normal and arthritic rats. J. Pharmacol. Exp. Ther. 326, 41–50 (2008).
Ohno, H. et al. The orally-active and selective c-Fms tyrosine kinase inhibitor Ki20227 inhibits disease progression in a collagen-induced arthritis mouse model. Eur. J. Immunol. 38, 283–291 (2008).
van Nimwegen, M. J. & van de Water, B. Focal adhesion kinase: a potential target in cancer therapy. Biochem. Pharmacol. 73, 597–609 (2007).
Miyazaki, T. et al. The role of c-Src kinase in the regulation of osteoclast function. Mod. Rheumatol. 16, 68–74 (2006).
Del Fattore, A. et al. Osteoclast receptors and signaling. Arch. Biochem. Biophys. 473, 147–160 (2008).
Wada, T. et al. RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 12, 17–25 (2006).
Shahrara, S. et al. Differential expression of the FAK family kinases in rheumatoid arthritis and osteoarthritis synovial tissues. Arthritis Res. Ther. 9, R112 (2007).
Takayanagi, H. et al. Suppression of arthritic bone destruction by adenovirus-mediated csk gene transfer to synoviocytes and osteoclasts. J. Clin. Invest. 104, 137–146 (1999).
Kay, J. & Calabrese, L. The role of interleukin-1 in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxford) 43 (Suppl. 3), iii2–iii9 (2004).
Segal, B. et al. Tumor necrosis factor (TNF) inhibitor therapy for rheumatoid arthritis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 106, 778–787 (2008).
Kumkumian, G. K. et al. Platelet-derived growth factor and IL-1 interactions in rheumatoid arthritis. Regulation of synoviocyte proliferation, prostaglandin production, and collagenase transcription. J. Immunol. 143, 833–837 (1989).
Campbell, I. K. et al. Production of macrophage colony-stimulating factor (M-CSF) by human articular cartilage and chondrocytes. Modulation by interleukin-1 and tumor necrosis factor alpha. Biochim. Biophys. Acta 1182, 57–63 (1993).
Miyachi, K. et al. Efficacy of imatinib mesylate (STI571) treatment for a patient with rheumatoid arthritis developing chronic myelogenous leukemia. Clin. Rheumatol. 22, 329–332 (2003).
Eklund, K. K. & Joensuu, H. Treatment of rheumatoid arthritis with imatinib mesylate: clinical improvement in three refractory cases. Ann. Med. 35, 362–367 (2003).
Walker, J. G. et al. Changes in synovial tissue Jak–STAT expression in rheumatoid arthritis in response to successful DMARD treatment. Ann. Rheum. Dis. 65, 1558–1564 (2006).
Pfizer Pipeline: as of September 30, 2008. http://media.pfizer.com/files/research/pipeline/2008_0930/pipeline_2008_0930.pdf (2008).
Incyte Corporation. Press Release: Incyte's JAK inhibitor demonstrates rapid and marked clinical improvement in rheumatoid arthritis patients. http://investor.incyte.com/phoenix.zhtml?c=69764&p=IROL-NewsText&t=Regular&id=1217246& (2008).
Cha, H. S. et al. A novel spleen tyrosine kinase inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. J. Pharmacol. Exp. Ther. 317, 571–578 (2006).
Weinblatt, M. E. et al. Treatment of rheumatoid arthritis with a syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 58, 3309–3318 (2008).
Firestein, G. S. Rheumatoid arthritis in a mouse? Nat. Clin. Pract. Rheumatol. 5, 1 (2009).
Alvarez, R. H. et al. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin. Proc. 81, 1241–1257 (2006).
Naito, M. Macrophage differentiation and function in health and disease. Pathol. Int. 58, 143–155 (2008).
Acknowledgements
We thank members of the Robinson laboratory for their scientific input. This work was funded by NIH NHLBI contract N01 HV 28183, NIH NIAMS R01 AR-054822, and Veterans Affairs Health Care System funding to W. H. Robinson; Stanford University Program in Immunology training grant support to C. D'Aura Swanson; and an NIH F31 Fellowship Award and a Lieberman Fellowship Award to R. T. Paniagua.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
D'Aura Swanson, C., Paniagua, R., Lindstrom, T. et al. Tyrosine kinases as targets for the treatment of rheumatoid arthritis. Nat Rev Rheumatol 5, 317–324 (2009). https://doi.org/10.1038/nrrheum.2009.82
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.82
This article is cited by
-
Development of piperidinyl dipyrrrolopyridine-based dual inhibitors of Janus kinase and Bruton’s tyrosine kinase: a potential therapeutic probability to deal with rheumatoid arthritis
Journal of Molecular Modeling (2020)
-
Multi-tissue network analysis for drug prioritization in knee osteoarthritis
Scientific Reports (2019)
-
Discovery of a highly selective JAK3 inhibitor for the treatment of rheumatoid arthritis
Scientific Reports (2018)
-
Importance of the novel organic cation transporter 1 for tyrosine kinase inhibition by saracatinib in rheumatoid arthritis synovial fibroblasts
Scientific Reports (2017)
-
Compensatory anabolic signaling in the sarcopenia of experimental chronic arthritis
Scientific Reports (2017)