Abstract
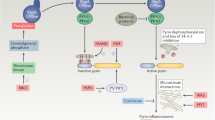
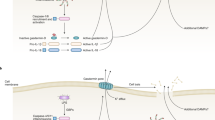
Evidence strongly suggests that excessive or protracted signaling, or both, by cell-surface or intracellular innate immune receptors is central to the pathogenesis of most autoimmune and autoinflammatory rheumatic diseases. The initiation of aberrant innate and adaptive immune responses in autoimmune diseases can be triggered by microbes and, at times, by endogenous molecules—particularly nucleic acids and related immune complexes—under sterile conditions. By contrast, most autoinflammatory syndromes are generally dependent on germline or de novo gene mutations that cause or facilitate inflammasome assembly. The consequent production of proinflammatory cytokines, principally interferon-α/β and tumor necrosis factor in autoimmune diseases, and interleukin-1β in autoinflammatory diseases, leads to the creation of autoamplification feedback loops and chronicity of these syndromes. These findings have resulted in a critical reappraisal of pathogenetic mechanisms, and provide a basis for the development of novel diagnostic and therapeutic modalities for these diseases.
Key Points
-
Toll-like receptors (TLRs), retinoid acid inducible gene-I-like receptors (RLRs) and nucleotide-binding and oligomerization domain-like receptors (NLRs) can detect the presence of pathogens and products of damaged tissues
-
Responses by these receptors usually benefit the host, but when inappropriately engaged by self molecules, or insufficiently inhibited, they can cause long-lasting immunopathology
-
In certain autoimmune rheumatic diseases, such as systemic lupus erythematosus, recognition of self nucleic acids by TLRs seems to be the major pathogenetic mechanism
-
In other diseases, such as rheumatoid arthritis and Sjögren's syndrome, recognition of products from microbes and damaged tissues by these or other innate sensors are likely to contribute
-
In autoinflammatory diseases, uncontrollable activation of the innate immune system is caused by mutations in components of the NLR system leading to inflammasome induction
-
These findings explain the efficacy of blocking proinflammatory cytokines in these diseases, and suggest that additional therapeutic targets will be identified within the signaling pathways of the innate immune sensors
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Baccala, R. et al. Sensors of the innate immune system: their mode of action. Nat. Rev. Rheumatol. 5, 448–456 (2009).
Ishii, K. J. & Akira, S. TLR ignores methylated RNA? Immunity 23, 111–113 (2005).
Schlee, M. et al. Approaching the RNA ligand for RIG-I? Immunol. Rev. 227, 66–74 (2009).
Bave, U., Vallin, H., Alm, G. V. & Ronnblom, L. Activation of natural interferon-alpha producing cells by apoptotic U937 cells combined with lupus IgG and its regulation by cytokines. J. Autoimmun. 17, 71–80 (2001).
Leadbetter, E. A. et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Lovgren, T., Eloranta, M. L., Bave, U., Alm, G. V. & Ronnblom, L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 50, 1861–1872 (2004).
Marshak-Rothstein, A. & Rifkin, I. R. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu. Rev. Immunol. 25, 419–441 (2007).
Baccala, R., Hoebe, K., Kono, D. H., Beutler, B. & Theofilopoulos, A. N. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat. Med. 13, 543–551 (2007).
Boule, M. W. et al. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J. Exp. Med. 199, 1631–1640 (2004).
Tian, J. et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 8, 487–496 (2007).
Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007).
Christensen, S. R. et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25, 417–428 (2006).
Pisitkun, P. et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science 312, 1669–1672 (2006).
Subramanian, S. et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl Acad. Sci. USA 103, 9970–9975 (2006).
Deane, J. A. et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810 (2007).
Santiago-Raber, M. L. et al. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J. Immunol. 181, 1556–1562 (2008).
Fairhurst, A. M. et al. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur. J. Immunol. 38, 1971–1978 (2008).
Theofilopoulos, A. N. & Dixon, F. J. Murine models of systemic lupus erythematosus. Adv. Immunol. 37, 269–390 (1985).
Kono, D. H. et al. Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc. Natl Acad. Sci. USA 106, 12061–12066 (2009).
Kim, Y. M., Brinkmann, M. M., Paquet, M. E. & Ploegh, H. L. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452, 234–238 (2008).
Banchereau, J. & Pascual, V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383–392 (2006).
Santer, D. M., Yoshio, T., Minota, S., Moller, T. & Elkon, K. B. Potent induction of IFN-alpha and chemokines by autoantibodies in the cerebrospinal fluid of patients with neuropsychiatric lupus. J. Immunol. 182, 1192–1201 (2009).
Harley, I. T., Kaufman, K. M., Langefeld, C. D., Harley, J. B. & Kelly, J. A. Genetic susceptibility to SLE: new insights from fine mapping and genome-wide association studies. Nat. Rev. Genet. 10, 285–290 (2009).
Graham, R. R., Hom, G., Ortmann, W. & Behrens, T. W. Review of recent genome-wide association scans in lupus. J. Intern. Med. 265, 680–688 (2009).
Zhuang, H. et al. Lupus-like disease and high interferon levels corresponding to trisomy of the type I interferon cluster on chromosome 9p. Arthritis Rheum. 54, 1573–1579 (2006).
Lee, P. Y. et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J. Exp. Med. 205, 2995–3006 (2008).
Lemke, G. & Rothlin, C. V. Immunobiology of the TAM receptors. Nat. Rev. Immunol. 8, 327–336 (2008).
Lech, M. et al. Tir8/Sigirr prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J. Exp. Med. 205, 1879–1888 (2008).
Baccala, R., Kono, D. H. & Theofilopoulos, A. N. Interferons as pathogenic effectors in autoimmunity. Immunol. Rev. 204, 9–26 (2005).
Theofilopoulos, A. N., Baccala, R., Beutler, B. & Kono, D. H. Type 1 interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 23, 307–335 (2005).
Janssen, E. et al. Efficient T cell activation via a Toll-Interleukin 1 Receptor-independent pathway. Immunity 24, 787–799 (2006).
Viorritto, I. C., Nikolov, N. P. & Siegel, R. M. Autoimmunity versus tolerance: can dying cells tip the balance? Clin. Immunol. 122, 125–134 (2007).
Lewis, M. J. & Botto, M. Complement deficiencies in humans and animals: links to autoimmunity. Autoimmunity 39, 367–378 (2006).
Mevorach, D., Zhou, J. L., Song, X. & Elkon, K. B. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J. Exp. Med. 188, 387–392 (1998).
Denny, M. F. et al. Accelerated macrophage apoptosis induces autoantibody formation and organ damage in systemic lupus erythematosus. J. Immunol. 176, 2095–2104 (2006).
Takemura, Y. et al. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J. Clin. Invest. 117, 375–386 (2007).
Allam, R. et al. Viral 5′-triphosphate RNA and non-CpG DNA aggravate autoimmunity and lupus nephritis via distinct TLR-independent immune responses. Eur. J. Immunol. 38, 3487–3498 (2008).
Yasutomo, K. et al. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat. Genet. 28, 313–314 (2001).
Napirei, M. et al. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat. Genet. 25, 177–181 (2000).
Nagata, S. Rheumatoid polyarthritis caused by a defect in DNA degradation. Cytokine Growth Factor Rev. 19, 295–302 (2008).
Stetson, D. B., Ko, J. S., Heidmann, T. & Medzhitov, R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598 (2008).
Rice, G. I. et al. Mutations involved in Aicardi–Goutieres syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41, 829–832 (2009).
Feldmeyer, L. et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr. Biol. 17, 1140–1145 (2007).
Schorlemmer, H. U., Kanzy, E. J., Langner, K. D. & Kurrle, R. Immunoregulation of SLE-like disease by the IL-1 receptor: disease modifying activity on BDF1 hybrid mice and MRL autoimmune mice. Agents Actions 39 (Spec. No.), C117–C120 (1993).
Bossu, P. et al. IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease. Proc. Natl Acad. Sci. USA 100, 14181–14186 (2003).
Rozzo, S. J. et al. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity 15, 435–443 (2001).
Croker, B. A. et al. Inflammation and autoimmunity caused by a SHP1 mutation depend on IL-1, MyD88, and a microbial trigger. Proc. Natl Acad. Sci. USA 105, 15028–15033 (2008).
Beutler, B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol. Rev. 227, 248–263 (2009).
Seibl, R. et al. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am. J. Pathol. 162, 1221–1227 (2003).
Kyburz, D. et al. Bacterial peptidoglycans but not CpG oligodeoxynucleotides activate synovial fibroblasts by toll-like receptor signaling. Arthritis Rheum. 48, 642–650 (2003).
Iwahashi, M. et al. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 50, 1457–1467 (2004).
Roelofs, M. F. et al. Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4 mediated synovial inflammation in rheumatoid arthritis (RA). Ann. Rheum. Dis. 68, 1486–1493 (2009).
Sacre, S. M. et al. Inhibitors of TLR8 reduce TNF production from human rheumatoid synovial membrane cultures. J. Immunol. 181, 8002–8009 (2008).
Herlands, R. A., Christensen, S. R., Sweet, R. A., Hershberg, U. & Shlomchik, M. J. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity 29, 249–260 (2008).
Shih, F. F., Racz, J. & Allen, P. M. Differential MHC class II presentation of a pathogenic autoantigen during health and disease. J. Immunol. 176, 3438–3448 (2006).
Choe, J. Y., Crain, B., Wu, S. R. & Corr, M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. J. Exp. Med. 197, 537–542 (2003).
Joosten, L. A. et al. Toll-like receptor 2 pathway drives streptococcal cell wall-induced joint inflammation: critical role of myeloid differentiation factor 88. J. Immunol. 171, 6145–6153 (2003).
Abdollahi-Roodsaz, S. et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–216 (2008).
Yoshitomi, H. et al. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201, 949–960 (2005).
Asagiri, M. et al. Cathepsin K-dependent toll-like receptor 9 signaling revealed in experimental arthritis. Science 319, 624–627 (2008).
Ewald, S. E. et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 456, 658–662 (2008).
Park, B. et al. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat. Immunol. 9, 1407–1414 (2008).
Okabe, Y., Sano, T. & Nagata, S. Regulation of the innate immune response by threonine-phosphatase of Eyes absent. Nature 460, 520–524 (2009).
Deng, G. M., Nilsson, I. M., Verdrengh, M., Collins, L. V. & Tarkowski, A. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat. Med. 5, 702–705 (1999).
Collins, L. V., Hajizadeh, S., Holme, E., Jonsson, I. M. & Tarkowski, A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 75, 995–1000 (2004).
Rossol, M. et al. Homozygosity for DNASE2 single-nucleotide polymorphisms in the 5′ regulatory region is associated with rheumatoid arthritis. Ann. Rheum. Dis. 68, 1498–1503 (2009).
Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6, 823–835 (2006).
Midwood, K. et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 15, 774–780 (2009).
Spachidou, M. P. et al. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjogren's syndrome. Clin. Exp. Immunol. 147, 497–503 (2007).
Kawakami, A. et al. Toll-like receptor in salivary glands from patients with Sjogren's syndrome: functional analysis by human salivary gland cell line. J. Rheumatol. 34, 1019–1026 (2007).
Ittah, M. et al. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-I IFN-dependent and -independent pathways. Eur. J. Immunol. 38, 1058–1064 (2008).
Bave, U. et al. Activation of the type I interferon system in primary Sjogren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 52, 1185–1195 (2005).
Gottenberg, J. E. et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren's syndrome. Proc. Natl Acad. Sci. USA 103, 2770–2775 (2006).
Nordmark, G., Alm, G. V. & Ronnblom, L. Mechanisms of Disease: primary Sjogren's syndrome and the type I interferon system. Nat. Clin. Pract. Rheumatol. 2, 262–269 (2006).
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J. Exp. Med. 202, 135–143 (2005).
Baechler, E. C. et al. An interferon signature in the peripheral blood of dermatomyositis patients is associated with disease activity. Mol. Med. 13, 59–68 (2007).
Eloranta, M. L. et al. A possible mechanism for endogenous activation of the type I interferon system in myositis patients with anti-Jo-1 or anti-Ro 52/anti-Ro 60 autoantibodies. Arthritis Rheum. 56, 3112–3124 (2007).
Masters, S. L., Simon, A., Aksentijevich, I. & Kastner, D. L. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu. Rev. Immunol. 27, 621–668 (2009).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 (2001).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411, 603–606 (2001).
Miceli-Richard, C. et al. CARD15 mutations in Blau syndrome. Nat. Genet. 29, 19–20 (2001).
Kanazawa, N. et al. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood 105, 1195–1197 (2005).
Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A. & Kolodner, R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 29, 301–305 (2001).
Aksentijevich, I. et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 46, 3340–3348 (2002).
Feldmann, J. et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71, 198–203 (2002).
Lachmann, H. J. et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N. Engl. J. Med. 360, 2416–2425 (2009).
Richards, N. et al. Interaction between pyrin and the apoptotic speck protein (ASC) modulates ASC-induced apoptosis. J. Biol. Chem. 276, 39320–39329 (2001).
Chae, J. J. et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc. Natl Acad. Sci. USA 103, 9982–9987 (2006).
Papin, S. et al. The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1beta processing. Cell Death Differ. 14, 1457–1466 (2007).
Yu, J. W. et al. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 13, 236–249 (2006).
Mansfield, E. et al. The familial Mediterranean fever protein, pyrin, associates with microtubules and colocalizes with actin filaments. Blood 98, 851–859 (2001).
Wise, C. A. et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum. Mol. Genet. 11, 961–969 (2002).
Shoham, N. G. et al. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl Acad. Sci. USA 100, 13501–13506 (2003).
Badour, K. et al. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J. Exp. Med. 199, 99–112 (2004).
Yang, H. & Reinherz, E. L. CD2BP1 modulates CD2-dependent T cell activation via linkage to protein tyrosine phosphatase (PTP)-PEST. J. Immunol. 176, 5898–5907 (2006).
Baum, W. et al. Binding of the intracellular Fas ligand (FasL) domain to the adaptor protein PSTPIP results in a cytoplasmic localization of FasL. J. Biol. Chem. 280, 40012–40024 (2005).
Dierselhuis, M. P., Frenkel, J., Wulffraat, N. M. & Boelens, J. J. Anakinra for flares of pyogenic arthritis in PAPA syndrome. Rheumatology (Oxford) 44, 406–408 (2005).
El-Shanti, H. I. & Ferguson, P. J. Chronic recurrent multifocal osteomyelitis: a concise review and genetic update. Clin. Orthop. Relat. Res. 462, 11–19 (2007).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
McGonagle, D. et al. Management of treatment resistant inflammation of acute on chronic tophaceous gout with anakinra. Ann. Rheum. Dis. 66, 1683–1684 (2007).
Jeru, I. et al. Mutations in NALP12 cause hereditary periodic fever syndromes. Proc. Natl Acad. Sci. USA 105, 1614–1619 (2008).
Pascual, V., Allantaz, F., Arce, E., Punaro, M. & Banchereau, J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 201, 1479–1486 (2005).
Church, L. D., Cook, G. P. & McDermott, M. F. Primer: inflammasomes and interleukin 1beta in inflammatory disorders. Nat. Clin. Pract. Rheumatol. 4, 34–42 (2008).
McDermott, M. F. et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97, 133–144 (1999).
Lobito, A. A. et al. Abnormal disulfide-linked oligomerization results in ER retention and altered signaling by TNFR1 mutants in TNFR1-associated periodic fever syndrome (TRAPS). Blood 108, 1320–1327 (2006).
Rebelo, S. L. et al. Modeling of tumor necrosis factor receptor superfamily 1A mutants associated with tumor necrosis factor receptor-associated periodic syndrome indicates misfolding consistent with abnormal function. Arthritis Rheum. 54, 2674–2687 (2006).
Acknowledgements
The work of the authors is supported by National Institute of Health grants. Space limitations precluded citation of many original publications, and we apologize for these omissions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Theofilopoulos, A., Gonzalez-Quintial, R., Lawson, B. et al. Sensors of the innate immune system: their link to rheumatic diseases. Nat Rev Rheumatol 6, 146–156 (2010). https://doi.org/10.1038/nrrheum.2009.278
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.278
This article is cited by
-
Innate immune memory in inflammatory arthritis
Nature Reviews Rheumatology (2023)
-
Monogenic autoinflammatory disease-associated cardiac damage
Inflammation Research (2023)
-
BANK1 interacts with TRAF6 and MyD88 in innate immune signaling in B cells
Cellular & Molecular Immunology (2020)
-
Familial Mediterranean fever is associated with a wide spectrum of inflammatory disorders: results from a large cohort study
Rheumatology International (2020)
-
Familial Mediterranean fever and atherosclerosis in childhood and adolescence
Rheumatology International (2020)