Abstract
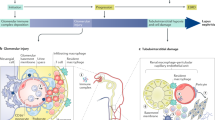
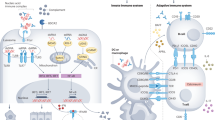
Lupus nephritis is a challenging clinical condition for which current therapies are unsatisfactory with respect to both remission induction and unwanted toxic effects. Despite intervention, the rates of end-stage renal disease seem to be increasing in the USA. Discoveries over the past decade have greatly improved our understanding of immune activation and effector inflammatory pathways in lupus nephritis; however, this increased understanding has not yet translated into the approval of an effective new therapeutic agent. An analysis of the mechanisms of action of novel immunomodulatory drugs in multiple models of murine lupus clearly shows that interacting networks of immune and effector pathways are recruited as the disease progresses. Reversing established disease by targeting a single cell population or inflammatory pathway is, therefore, difficult once long-lived autoreactive lymphocyte populations are present and peripheral organs are inflamed. Data from murine models of lupus suggest that we need to consider new paradigms for the management of systemic lupus erythematosus that include earlier immune intervention, long-term maintenance therapies and protection of target organs.
Key Points
-
The incidence of end-stage renal disease resulting from lupus nephritis is static or increasing in the USA
-
Several high-profile clinical trials of novel biologic therapies for lupus nephritis that target the immune system have failed despite convincing evidence that lupus nephritis is immune-mediated
-
Although inhibition of single cell types or inflammatory mediators can prevent disease in murine models of lupus, the efficacy of this approach is substantially lower in established disease
-
Multiple interactions between innate and adaptive immune pathways in established disease amplify inflammation and make it difficult to restore normal tolerance
-
An understanding of the pathways that mediate intrinsic renal cell activation, interstitial renal inflammation, renal hypoxia and fibrosis should yield new strategies for protection of the kidneys
-
The role of genetic polymorphisms in determining susceptibility to systemic lupus erythematosus and to renal damage might enable targeting of therapies to particular subgroups of patients
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sidiropoulos, P. I., Kritikos, H. D. & Boumpas, D. T. Lupus nephritis flares. Lupus 14, 49–52 (2005).
Mok, C. C. et al. Predictors and outcome of renal flares after successful cyclophosphamide treatment for diffuse proliferative lupus glomerulonephritis. Arthritis Rheum. 50, 2559–2568 (2004).
Appel, G. B. et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J. Am. Soc. Nephrol. 20, 1103–1112 (2009).
Mok, C. C. Therapeutic options for resistant lupus nephritis. Semin. Arthritis Rheum. 36, 71–81 (2006).
Dooley, M. A. in Dubois' Lupus Erythematosus 7th edn (eds Wallace, D. J. & Hahn, H. B.) 1112–1130 (Lippincott Williams & Wilkins, Philadelphia, 2007).
Chan, T. M. Preventing renal failure in patients with severe lupus nephritis. Kidney Int. 67 (Suppl.), S116–S119 (2005).
Contreras, G. et al. Sequential therapies for proliferative lupus nephritis. N. Engl. J. Med. 350, 971–980 (2004).
Contreras, G., Tozman, E., Nahar, N. & Metz, D. Maintenance therapies for proliferative lupus nephritis: mycophenolate mofetil, azathioprine and intravenous cyclophosphamide. Lupus 14 (Suppl. 1), S33–S38 (2005).
Dooley, M. A., Hogan, S., Jennette, C. & Falk, R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Glomerular Disease Collaborative Network. Kidney Int. 51, 1188–1195 (1997).
Costenbader, K. H., Solomon, D. H., Winkelmayer, W. & Brookhart, M. A. Incidence of end-stage renal disease due to lupus nephritis in the US, 1995–2004 [abstract 1927]. Arthritis Rheum. 58 (Suppl.), S872 (2008).
Wiesendanger, M., Stanevsky, A., Kovsky, S. & Diamond, B. Novel therapeutics for systemic lupus erythematosus. Curr. Opin. Rheumatol. 18, 227–235 (2006).
Clynes, R., Dumitru, C. & Ravetch, J. V. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 279, 1052–1054 (1998).
Turnberg, D. & Cook, H. T. Complement and glomerulonephritis: new insights. Curr. Opin. Nephrol. Hypertens. 14, 223–228 (2005).
Anders, H. J. & Schlondorff, D. Toll-like receptors: emerging concepts in kidney disease. Curr. Opin. Nephrol. Hypertens. 16, 177–183 (2007).
Segerer, S. & Schlondorff, D. B cells and tertiary lymphoid organs in renal inflammation. Kidney Int. 73, 533–537 (2008).
Jeruc, J. et al. Tubulo-interstitial involvement in lupus nephritis with emphasis on pathogenesis. Wien. Klin. Wochenschr. 112, 702–706 (2000).
Chan, O. T., Hannum, L. G., Haberman, A. M., Madaio, M. P. & Shlomchik, M. J. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 189, 1639–1648 (1999).
Schwartz, M. M. The pathology of lupus nephritis. Semin. Nephrol. 27, 22–34 (2007).
Lewis, E. J. & Schwartz, M. M. Pathology of lupus nephritis. Lupus 14, 31–38 (2005).
Schlondorff, D. O. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney Int. 74, 860–866 (2008).
Schiffer, L. et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J. Immunol. 180, 1938–1947 (2008).
Ferraccioli, G. & Romano, G. Renal interstitial cells, proteinuria and progression of lupus nephritis: new frontiers for old factors. Lupus 17, 533–540 (2008).
Mazzone, M. et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 136, 839–851 (2009).
Kumpers, P. et al. The Tie2 receptor antagonist Angiopoietin-2 facilitates vascular inflammation in systemic lupus erythematosus. Ann. Rheum. Dis. 68, 1638–1643 (2008).
Yeboah, M. M. et al. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 74, 62–69 (2008).
Yao, G. et al. Evaluation of renal vascular lesions using circulating endothelial cells in patients with lupus nephritis. Rheumatology (Oxford) 47, 432–436 (2008).
Izmirly, P. M. et al. Expression of endothelial protein C receptor in cortical peritubular capillaries associates with a poor clinical response in lupus nephritis. Rheumatology (Oxford) 48, 513–519 (2009).
Sesin, C. A., Yin, X., Esmon, C. T., Buyon, J. P. & Clancy, R. M. Shedding of endothelial protein C receptor contributes to vasculopathy and renal injury in lupus: in vivo and in vitro evidence. Kidney Int. 68, 110–120 (2005).
Shankland, S. J. The podocyte's response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 69, 2131–2147 (2006).
Michaud, J. L. & Kennedy, C. R. The podocyte in health and disease: insights from the mouse. Clin. Sci. (Lond.) 112, 325–335 (2007).
Crowley, S. D. et al. Glomerular type 1 angiotensin receptors augment kidney injury and inflammation in murine autoimmune nephritis. J. Clin. Invest. 119, 943–953 (2009).
Duran-Barragan, S., McGwin, G., Jr, Vila, L. M., Reveille, J. D. & Alarcon, G. S. Angiotensin-converting enzyme inhibitors delay the occurrence of renal involvement and are associated with a decreased risk of disease activity in patients with systemic lupus erythematosus—results from LUMINA (LIX): a multiethnic US cohort. Rheumatology (Oxford) 47, 1093–1096 (2008).
Faurschou, M., Starklint, H., Halberg, P. & Jacobsen, S. Prognostic factors in lupus nephritis: diagnostic and therapeutic delay increases the risk of terminal renal failure. J. Rheumatol. 33, 1563–1569 (2006).
Mok, C. C. Prognostic factors in lupus nephritis. Lupus 14, 39–44 (2005).
Segerer, S. et al. Compartment specific expression of dendritic cell markers in human glomerulonephritis. Kidney Int. 74, 37–46 (2008).
Tucci, M. et al. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 58, 251–262 (2008).
Kruger, B., Schroppel, B. & Murphy, B. T. Genetic polymorphisms and the fate of the transplanted organ. Transplant Rev. (Orlando) 22, 131–140 (2008).
Hom, G. et al. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N. Engl. J. Med. 358, 900–909 (2008).
Warchol, T., Lianeri, M., Wudarski, M., Lacki, J. K. & Jagodzinski, P. P. IL-18 105 A>C polymorphism contributes to renal manifestations in patients with SLE. Rheumatol Int. doi: 10.1007/s00296-009-0934–0933.
Liu, K. et al. Kallikrein genes are associated with lupus and glomerular basement membrane-specific antibody-induced nephritis in mice and humans. J. Clin. Invest. 119, 911–923 (2009).
Phan, T. G., Green, J. A., Gray, E. E., Xu, Y. & Cyster, J. G. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 10, 786–793 (2009).
Mamula, M. J. Epitope spreading: the role of self peptides and autoantigen processing by B lymphocytes. Immunol. Rev. 164, 231–239 (1998).
Roosnek, E. & Lanzavecchia, A. Efficient and selective presentation of antigen-antibody complexes by rheumatoid factor B cells. J. Exp. Med. 173, 487–489 (1991).
Lund, F. E. Cytokine-producing B lymphocytes-key regulators of immunity. Curr. Opin. Immunol. 20, 332–338 (2008).
Ngo, V. N., Cornall, R. J. & Cyster, J. G. Splenic T zone development is B cell dependent. J. Exp. Med. 194, 1649–1660 (2001).
Cassese, G. et al. Inflamed kidneys of NZB/W mice are a major site for the homeostasis of plasma cells. Eur. J. Immunol. 31, 2726–2732 (2001).
Takemura, S. et al. Lymphoid neogenesis in rheumatoid synovitis. J. Immunol. 167, 1072–1080 (2001).
Odendahl, M. et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 165, 5970–5979 (2000).
Huang, W. et al. The effect of anti-CD40 ligand antibody on B cells in human SLE. Arthritis Rheum. 46, 1554–1562 (2002).
Merrill, J. T. et al. Efficacy and safety of rituximab in patients with moderately to severely active systemic lupus erythematosus (SLE): Results from the randomized, double-blind phase II/III study EXPLORER [abstract L12]. Presented at the American College of Rheumatology Annual Meeting, 2008 October, San Francisco, CA.
Furie, R. et al. Efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR Study [abstract 1149]. Arthritis Rheum. 60 (Suppl.), S429 (2009).
Anolik, J. H. et al. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis Rheum. 50, 3580–3590 (2004).
Vallerskog, T. et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 8, R167 (2006).
Ahuja, A. et al. Depletion of B cells in murine lupus: efficacy and resistance. J. Immunol. 179, 3351–3361 (2007).
Ramanujam, M. et al. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J. Immunol. 173, 3524–3534 (2004).
Thien, M. et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20, 785–798 (2004).
Scholz, J. L. et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc. Natl Acad. Sci. USA 105, 15517–15522 (2008).
Doreau, A. et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat. Immunol. 10, 778–785 (2009).
Ramanujam, M. et al. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J. Clin. Invest. 116, 724–734 (2006).
Lai Kwan Lam, Q., King Hung Ko, O., Zheng, B. J. & Lu, L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc. Natl Acad. Sci. USA 105, 14993–14998 (2008).
Ramanujam, M. & Davidson, A. BAFF blockade for systemic lupus erythematosus: will the promise be fulfilled? Immunol. Rev. 223, 156–174 (2008).
Jacob, C. O. et al. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J. Immunol. 177, 2671–2680 (2006).
Wallace, D. J. et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 61, 1168–1178 (2009).
Navarra, S. et al. Belimumab, a BLyS-specific inhibitor, reduced disease activity, flares and prednisone use in patients with active SLE: efficacy and safety results from the Phase 3 BLISS-52 Study [abstract LB1]. Presented at the American College of Rheumatology Annual Meeting, 2009 October, Philadelphia, PA.
Chen, Y., Park, Y. B., Patel, E. & Silverman, G. J. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J. Immunol. 182, 6031–6043 (2009).
Manson, J. J., Mauri, C. & Ehrenstein, M. R. Natural serum IgM maintains immunological homeostasis and prevents autoimmunity. Springer Semin. Immunopathol. 26, 425–432 (2005).
Mihara, M. et al. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. J. Clin. Invest. 106, 91–101 (2000).
Schiffer, L. et al. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J. Immunol. 171, 489–497 (2003).
Linterman, M. A. et al. Roquin differentiates the specialized functions of duplicated T cell costimulatory receptor genes CD28 and ICOS. Immunity 30, 228–241 (2009).
Ettinger, R. et al. IL-21 and BAFF/BLyS synergize in stimulating plasma cell differentiation from a unique population of human splenic memory B cells. J. Immunol. 178, 2872–2882 (2007).
Bubier, J. A. et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl Acad. Sci. USA 106, 1518–1523 (2009).
Linterman, M. A. et al. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 206, 561–576 (2009).
Kalled, S. L., Cutler, A. H., Datta, S. K. & Thomas, D. W. Anti-CD40 ligand antibody treatment of SNF1 mice with established nephritis: preservation of kidney function. J. Immunol. 160, 2158–2165 (1998).
Forestier, C. et al. Expansion and hyperactivity of CD1d-restricted NKT cells during the progression of systemic lupus erythematosus in (New Zealand Black x New Zealand White) F1 mice. J. Immunol. 175, 763–770 (2005).
Scalapino, K. J. & Daikh, D. I. Suppression of glomerulonephritis in NZB/NZW lupus prone mice by adoptive transfer of ex vivo expanded regulatory T cells. PLoS ONE 4, e6031 (2009).
Beriou, G. et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood 113, 4240–4249 (2009).
Wu, H. Y., Center, E. M., Tsokos, G. C. & Weiner, H. L. Suppression of murine SLE by oral anti-CD3: inducible CD4+CD25-LAP+ regulatory T cells control the expansion of IL-17+ follicular helper T cells. Lupus 18, 586–596 (2009).
Perl, A. et al. T-cell and B-cell signaling biomarkers and treatment targets in lupus. Curr. Opin. Rheumatol. 21, 454–464 (2009).
Singh, R. P., Hahn, B. H. & La Cava, A. Tuning immune suppression in systemic autoimmunity with self-derived peptides. Inflamm. Allergy Drug Targets 7, 253–259 (2008).
Kang, H. K., Liu, M. & Datta, S. K. Low-dose peptide tolerance therapy of lupus generates plasmacytoid dendritic cells that cause expansion of autoantigen-specific regulatory T cells and contraction of inflammatory Th17 cells. J. Immunol. 178, 7849–7858 (2007).
Sharabi, A. & Mozes, E. The suppression of murine lupus by a tolerogenic peptide involves foxp3-expressing CD8 cells that are required for the optimal induction and function of foxp3-expressing CD4 cells. J. Immunol. 181, 3243–3251 (2008).
United States National Library of Medicine NIH. ClinicalTrials.gov [online], (2009).
Pascual, V., Farkas, L. & Banchereau, J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr. Opin. Immunol. 18, 676–682 (2006).
Mathian, A., Weinberg, A., Gallegos, M., Banchereau, J. & Koutouzov, S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice. J. Immunol. 174, 2499–2506 (2005).
Zagury, D. et al. IFNalpha kinoid vaccine-induced neutralizing antibodies prevent clinical manifestations in a lupus flare murine model. Proc. Natl Acad. Sci. USA 106, 5294–5299 (2009).
Groom, J. R. et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J. Exp. Med. 204, 1959–1971 (2007).
Pawar, R. D. et al. Inhibition of Toll-like receptor-7 (TLR-7) or TLR-7 plus TLR-9 attenuates glomerulonephritis and lung injury in experimental lupus. J. Am. Soc. Nephrol. 18, 1721–1731 (2007).
Ehlers, M. & Ravetch, J. V. Opposing effects of Toll-like receptor stimulation induce autoimmunity or tolerance. Trends Immunol. 28, 74–79 (2007).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Lafyatis, R., York, M. & Marshak-Rothstein, A. Antimalarial agents: closing the gate on Toll-like receptors? Arthritis Rheum. 54, 3068–3070 (2006).
Crispin, J. C., Kyttaris, V. C., Juang, Y. T. & Tsokos, G. C. How signaling and gene transcription aberrations dictate the systemic lupus erythematosus T cell phenotype. Trends Immunol. 29, 110–115 (2008).
Gilliet, M., Cao, W. & Liu, Y. J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8, 594–606 (2008).
Chaturvedi, A., Dorward, D. & Pierce, S. K. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity 28, 799–809 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C. Aranow declares that she has acted as a consultant for and/or received research support from the following companies: Amgen, Bristol-Myers Squibb, Genentech, Human Genome Sciences, La Jolla Pharmaceutical, Medimmune, Novo Nordisk and UCB.
Rights and permissions
About this article
Cite this article
Davidson, A., Aranow, C. Lupus nephritis: lessons from murine models. Nat Rev Rheumatol 6, 13–20 (2010). https://doi.org/10.1038/nrrheum.2009.240
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.240
This article is cited by
-
Circular RNA-0007059 protects cell viability and reduces inflammation in a nephritis cell model by inhibiting microRNA-1278/SHP-1/STAT3 signaling
Molecular Medicine (2021)
-
Clinicopathological factors for tubulointerstitial injury in lupus nephritis
Clinical Rheumatology (2020)
-
MicroRNA-155 Suppresses Mesangial Cell Proliferation and TGF-β1 Production via Inhibiting CXCR5-ERK Signaling Pathway in Lupus Nephritis
Inflammation (2019)
-
What is damaging the kidney in lupus nephritis?
Nature Reviews Rheumatology (2016)
-
NF-κB in inflammation and renal diseases
Cell & Bioscience (2015)