Abstract
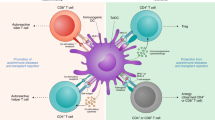
Dendritic cells (DCs) are central in inducing immunity and in mediating immune tolerance in their role as professional antigen-presenting cells. In the absence of DCs, a fatal autoimmunity develops in animal models. Although the role of DCs has been investigated extensively in the pathogenesis of rheumatoid arthritis (RA), it remains unclear whether DCs initiate autoimmunity in this disease. Nevertheless, evidence points towards a significant role for DCs in disease maintenance and progression. Current biologic therapies target cytokine products of antigen-presenting cells, such as tumor necrosis factor, interleukin-1 and interleukin-6. Emerging therapies for RA exploit the tolerogenic capacity of DCs. 'Tolerogenic' DCs can be generated from myeloid precursors ex vivo, loaded with antigen, and manipulated to suppress autoimmune responses in vivo, through the induction of activation-induced cell death, anergy, and/or regulatory T cells. Cells that are primed by DCs, such as B cells, type 1 and type 17 T helper cells, and that have been implicated in certain models of autoimmunity, are also being considered as additional targets for immune-based therapy. Studies to validate these approaches to ameliorate autoimmunity will be necessary before their application in the clinic.
Key Points
-
Dendritic cells (DCs) are implicated in the pathogenesis of rheumatoid arthritis (RA)
-
These cells and their products, such as tumor necrosis factor, interleukin-1 and interleukin-6, localize in rheumatoid synovium
-
The proinflammatory products of DCs can be targeted to ameliorate RA
-
DCs can also be manipulated to induce tolerance in animal models; studies are underway to test their tolerogenic activity in patients with RA
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wenink, M. H., Han, W., Toes, R. E. & Radstake, T. R. Dendritic cells and their potential implication in pathology and treatment of rheumatoid arthritis. Handb. Exp. Pharmacol. 188, 81–98 (2009).
Zhou, L., Chong, M. M. & Littman, D. R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009).
Ohnmacht, C. et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 206, 549–559 (2009).
Somersan, S. & Bhardwaj, N. Tethering and tickling: a new role for the phosphatidylserine receptor. J. Cell Biol. 155, 501–504 (2001).
Kobayashi, N. et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity 27, 927–940 (2007).
Skoberne, M., Beignon, A. S., Larsson, M. & Bhardwaj, N. Apoptotic cells at the crossroads of tolerance and immunity. Curr. Top. Microbiol. Immunol. 289, 259–292 (2005).
Skoberne, M. et al. The apoptotic-cell receptor CR3, but not αvβ5, is a regulator of human dendritic-cell immunostimulatory function. Blood 108, 947–955 (2006).
Thomas, R. et al. Dendritic cells and the pathogenesis of rheumatoid arthritis. J. Leukoc. Biol. 66, 286–292 (1999).
Pettit, A. R., MacDonald, K. P., O'Sullivan, B. & Thomas, R. Differentiated dendritic cells expressing nuclear RelB are predominantly located in rheumatoid synovial tissue perivascular mononuclear cell aggregates. Arthritis Rheum. 43, 791–800 (2000).
Zvaifler, N. J., Steinman, R. M., Kaplan, G., Lau, L. L. & Rivelis, M. Identification of immunostimulatory dendritic cells in the synovial effusions of patients with rheumatoid arthritis. J. Clin. Invest. 76, 789–800 (1985).
Jongbloed, S. L. et al. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis Res. Ther. 8, R15 (2006).
Santiago-Schwarz, F., Anand, P., Liu, S. & Carsons, S. E. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J. Immunol. 167, 1758–1768 (2001).
van Lieshout, A. W. et al. Inhibition of TNFα during maturation of dendritic cells results in the development of semi-mature cells: a potential mechanism for the beneficial effects of TNFα blockade in rheumatoid arthritis. Ann. Rheum. Dis. 64, 408–414 (2005).
Leung, B. P. et al. A novel dendritic cell-induced model of erosive inflammatory arthritis: distinct roles for dendritic cells in T cell activation and induction of local inflammation. J. Immunol. 169, 7071–7077 (2002).
Tsark, E. C. et al. Differential MHC class II-mediated presentation of rheumatoid arthritis autoantigens by human dendritic cells and macrophages. J. Immunol. 169, 6625–6633 (2002).
Steenbakkers, P. G. et al. Localization of MHC class II/human cartilage glycoprotein-39 complexes in synovia of rheumatoid arthritis patients using complex-specific monoclonal antibodies. J. Immunol. 170, 5719–5727 (2003).
Page, G. & Miossec, P. RANK and RANKL expression as markers of dendritic cell–T cell interactions in paired samples of rheumatoid synovium and lymph nodes. Arthritis Rheum. 52, 2307–2312 (2005).
Martin, C. A. et al. Aberrant extracellular and dendritic cell (DC) surface expression of heat shock protein (hsp)70 in the rheumatoid joint: possible mechanisms of hsp/DC-mediated cross-priming. J. Immunol. 171, 5736–5742 (2003).
Wing, K. et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 (2008).
Li, Y. & Begovich, A. B. Unraveling the genetics of complex diseases: susceptibility genes for rheumatoid arthritis and psoriasis. Semin. Immunol. doi: 10.1016/j.smim.2009.04.002.
Humby, F. et al. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 6, e1 (2009).
Klareskog, L., Catrina, A. I. & Paget, S. Rheumatoid arthritis. Lancet 373, 659–672 (2009).
Lebre, M. C. et al. Rheumatoid arthritis synovium contains two subsets of CD83−DC-LAMP− dendritic cells with distinct cytokine profiles. Am. J. Pathol. 172, 940–950 (2008).
van der Pouw Kraan, T. C. et al. Rheumatoid arthritis subtypes identified by genomic profiling of peripheral blood cells: assignment of a type I interferon signature in a subpopulation of patients. Ann. Rheum. Dis. 66, 1008–1014 (2007).
Chabaud, M., Fossiez, F., Taupin, J. L. & Miossec, P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J. Immunol. 161, 409–414 (1998).
Annunziato, F., Cosmi, L., Liotta, F., Maggi, E. & Romagnani, S. Type 17 T-helper cells—origins, features and possible roles in rheumatic disease. Nat. Rev. Rheumatol. 5, 325–331 (2009).
Murphy, C. A. et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1957 (2003).
Fossiez, F. et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593–2603 (1996).
Shahrara, S., Pickens, S. R., Dorfleutner, A. & Pope, R. M. IL-17 induces monocyte migration in rheumatoid arthritis. J. Immunol. 182, 3884–3891 (2009).
Kotake, S. et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 103, 1345–1352 (1999).
Koenders, M. I. et al. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am. J. Pathol. 167, 141–149 (2005).
Kirkham, B. W. et al. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort). Arthritis Rheum. 54, 1122–1131 (2006).
Yamada, H. et al. Th1 but not Th17 cells predominate in the joints of patients with rheumatoid arthritis. Ann. Rheum. Dis. 67, 1299–1304 (2008).
Chang, M. et al. The inflammatory disease-associated variants in IL12B and IL23R are not associated with rheumatoid arthritis. Arthritis Rheum. 58, 1877–1881 (2008).
Brentano, F. et al. Abundant expression of the interleukin (IL)23 subunit p19, but low levels of bioactive IL23 in the rheumatoid synovium: differential expression and Toll-like receptor-(TLR) dependent regulation of the IL23 subunits, p19 and p40, in rheumatoid arthritis. Ann. Rheum. Dis. 68, 143–150 (2009).
Wiekowski, M. T. et al. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 166, 7563–7570 (2001).
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Morelli, A. E. & Thomson, A. W. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat. Rev. Immunol. 7, 610–621 (2007).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Mahnke, K., Qian, Y., Knop, J. & Enk, A. H. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood 101, 4862–4869 (2003).
Fujikado, N. et al. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat. Med. 14, 176–180 (2008).
Balanescu, A. et al. Early and late effect of infliximab on circulating dendritic cells phenotype in rheumatoid arthritis patients. Int. J. Clin. Pharmacol. Res. 25, 9–18 (2005).
Dhodapkar, M. V., Steinman, R. M., Krasovsky, J., Munz, C. & Bhardwaj, N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193, 233–238 (2001).
Hill, J. A. et al. Cutting edge: the conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. J. Immunol. 171, 538–541 (2003).
Martin, E., O'Sullivan, B., Low, P. & Thomas, R. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10. Immunity 18, 155–167 (2003).
Lan, Y. Y. et al. “Alternatively activated” dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4–Ig. J. Immunol. 177, 5868–5877 (2006).
Bluestone, J. A., Thomson, A. W., Shevach, E. M. & Weiner, H. L. What does the future hold for cell-based tolerogenic therapy? Nat. Rev. Immunol. 7, 650–654 (2007).
Fallarino, F., Gizzi, S., Mosci, P., Grohmann, U. & Puccetti, P. Tryptophan catabolism in IDO+ plasmacytoid dendritic cells. Curr. Drug Metab. 8, 209–216 (2007).
Manches, O. et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J. Clin. Invest. 118, 3431–3439 (2008).
Chung, D. J. et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood 114, 555–563 (2009).
Bhardwaj, N. et al. IL-6/IFN-β2 in synovial effusions of patients with rheumatoid arthritis and other arthritides. Identification of several isoforms and studies of cellular sources. J. Immunol. 143, 2153–2159 (1989).
Genovese, M. C. et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 58, 2968–2980 (2008).
Stanczyk, J., Ospelt, C. & Gay, S. Is there a future for small molecule drugs in the treatment of rheumatic diseases? Curr. Opin. Rheumatol. 20, 257–262 (2008).
Oukka, M. Th17 cells in immunity and autoimmunity. Ann. Rheum. Dis. 67 (Suppl. 3), iii26–29 (2008).
Cohen, S. B. et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 58, 1299–1309 (2008).
Mellor, A. L. & Munn, D. H. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat. Rev. Immunol. 8, 74–80 (2008).
Criado, G., Simelyte, E., Inglis, J. J., Essex, D. & Williams, R. O. Indoleamine 2,3 dioxygenase-mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 60, 1342–1351 (2009).
Bianco, N. R., Kim, S. H., Ruffner, M. A. & Robbins, P. D. Therapeutic effect of exosomes from indoleamine 2,3-dioxygenase-positive dendritic cells in collagen-induced arthritis and delayed-type hypersensitivity disease models. Arthritis Rheum. 60, 380–389 (2009).
Acknowledgements
The author thanks Cynthia Magro, William St. Clair, Jonathan Poe, David Pisetsky, Karen Haas, Damian Maseda, and Takashi Matsushita for their assistance and suggestions. This work was supported by grants from the National Institutes of Health (AI56363, CA105001, CA96547, and AI057157).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Khan, S., Greenberg, J. & Bhardwaj, N. Dendritic cells as targets for therapy in rheumatoid arthritis. Nat Rev Rheumatol 5, 566–571 (2009). https://doi.org/10.1038/nrrheum.2009.185
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2009.185
This article is cited by
-
Mechanistic target of rapamycin (mTOR): a potential new therapeutic target for rheumatoid arthritis
Arthritis Research & Therapy (2023)
-
Disease-microenvironment modulation by bare- or engineered-exosome for rheumatoid arthritis treatment
Biomaterials Research (2023)
-
Vaccines prevent reinduction of rheumatoid arthritis symptoms in collagen-induced arthritis mouse model
Drug Delivery and Translational Research (2023)
-
A molecular insight of inflammatory cascades in rheumatoid arthritis and anti-arthritic potential of phytoconstituents
Molecular Biology Reports (2022)
-
The effect of the cholinergic anti-inflammatory pathway on collagen-induced arthritis involves the modulation of dendritic cell differentiation
Arthritis Research & Therapy (2018)