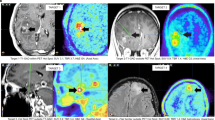
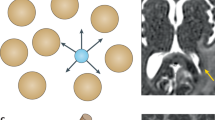
Key Points
-
Improvement of brain tumour diagnostics is necessary because therapies are extremely expensive and their efficient use is mandatory
-
Both amino acid PET and advanced MRI methods (perfusion-weighted imaging, diffusion-weighted imaging and magnetic resonance spectroscopic imaging) provide substantial additional information for brain tumour diagnostics
-
Advanced MRI methods are readily available but interpretation is challenging and images are frequently impaired by susceptibility artefacts
-
Amino acid PET requires additional scanning but is a robust and attractive approach for clinicians because of easy scan reading
-
Information from amino acid PET and advanced MRI methods seems to be complementary but multimodal studies are scarce and urgently needed
Abstract
Despite the fact that MRI has evolved to become the standard method for diagnosis and monitoring of patients with brain tumours, conventional MRI sequences have two key limitations: the inability to show the full extent of the tumour and the inability to differentiate neoplastic tissue from nonspecific, treatment-related changes after surgery, radiotherapy, chemotherapy or immunotherapy. In the past decade, PET involving the use of radiolabelled amino acids has developed into an important diagnostic tool to overcome some of the shortcomings of conventional MRI. The Response Assessment in Neuro-Oncology working group — an international effort to develop new standardized response criteria for clinical trials in brain tumours — has recommended the additional use of amino acid PET imaging for brain tumour management. Concurrently, a number of advanced MRI techniques such as magnetic resonance spectroscopic imaging and perfusion weighted imaging are under clinical evaluation to target the same diagnostic problems. This Review summarizes the clinical role of amino acid PET in relation to advanced MRI techniques for differential diagnosis of brain tumours; delineation of tumour extent for treatment planning and biopsy guidance; post-treatment differentiation between tumour progression or recurrence versus treatment-related changes; and monitoring response to therapy. An outlook for future developments in PET and MRI techniques is also presented.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 16 (Suppl. 4), iv1–iv63 (2014).
Nayak, L., Lee, E. Q. & Wen, P. Y. Epidemiology of brain metastases. Curr. Oncol. Rep. 14, 48–54 (2012).
Louis, D. N. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 131, 803–820 (2016).
Ohgaki, H. & Kleihues, P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J. Neuropathol. Exp. Neurol. 64, 479–489 (2005).
Albert, N. L. et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 18, 1199–1208 (2016). This study presents the recommendations of an international group of experts for the clinical use of PET imaging in brain tumours.
Ryken, T. C. et al. The role of imaging in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 118, 435–460 (2014).
Chung, C., Metser, U. & Menard, C. Advances in magnetic resonance imaging and positron emission tomography imaging for grading and molecular characterization of glioma. Semin. Radiat. Oncol. 25, 164–171 (2015).
Herholz, K., Coope, D. & Jackson, A. Metabolic and molecular imaging in neuro-oncology. Lancet Neurol. 6, 711–724 (2007).
Collet, S. et al. [18F]-fluoro-L-thymidine PET and advanced MRI for preoperative grading of gliomas. Neuroimage Clin. 8, 448–454 (2015).
Nowosielski, M. et al. An intra-individual comparison of MRI, [18F]-FET and [18F]-FLT PET in patients with high-grade gliomas. PLoS ONE 9, e95830 (2014).
Sollini, M. et al. Diagnostic performances of [18F]fluorocholine positron emission tomography in brain tumors. Q. J. Nucl. Med. Mol. Imaging 1 Sep 2015 [epub ahead of print] (2015).
Gerstner, E. et al. ACRIN 6684: assessment of tumor hypoxia in newly diagnosed GBM using 18F-FMISO PET and MRI. Clin. Cancer Res. 22, 5079–5086 (2016).
Kobayashi, H. et al. Usefulness of FMISO-PET for glioma analysis. Neurol. Med. Chir. (Tokyo) 53, 773–738 (2013).
Rapp, M. et al. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J. Nucl. Med. 54, 229–235 (2013).
Galldiks, N. et al. The use of dynamic O-(2-18F-fluoroethyl)-L-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 17, 1293–1300 (2015).
Rapp, M. et al. Clinical value of O-(2-[18F]-fluoroethyl)-L-tyrosine positron emission tomography in patients with low-grade glioma. Neurosurg. Focus 34, E3 (2013).
Singhal, T., Narayanan, T. K., Jain, V., Mukherjee, J. & Mantil, J. 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol. Imaging Biol. 10, 1–18 (2008).
Langen, K. J., Tonn, J. C., Weller, M. & Galldiks, N. Letter to the editor: “the role of imaging in the management of progressive glioblastoma. A systematic review and evidence-based clinical practice guideline” [J Neurooncol 2014; 118:435–460]. J. Neurooncol. 120, 665–666 (2014).
Swissmedic. Swiss Agency for Therapeutic Products. Swissmedic J. 13, 651 (2014).
Okubo, S. et al. Correlation of l-methyl-11C-methionine (MET) uptake with l-type amino acid transporter 1 in human gliomas. J. Neurooncol. 99, 217–225 (2010).
Youland, R. S. et al. The role of LAT1 in 18F-DOPA uptake in malignant gliomas. J. Neurooncol. 111, 11–18 (2013).
Habermeier, A. et al. System l amino acid transporter LAT1 accumulates O-(2-fluoroethyl)-L-tyrosine (FET). Amino Acids 47, 335–344 (2015).
Becherer, A. et al. Brain tumour imaging with PET: a comparison between [18F]fluorodopa and [11C]methionine. Eur. J. Nucl. Med. Mol. Imaging 30, 1561–1567 (2003).
Grosu, A. L. et al. An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int. J. Radiat. Oncol. Biol. Phys. 81, 1049–1058 (2011).
Kratochwil, C. et al. Intra-individual comparison of 18F-FET and 18F-DOPA in PET imaging of recurrent brain tumors. Neuro Oncol. 16, 434–440 (2014).
Calcagni, M. L. et al. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine (F-18 FET) PET for glioma grading: assessment of individual probability of malignancy. Clin. Nucl. Med. 36, 841–847 (2011).
Pöpperl, G. et al. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur. J. Nucl. Med. Mol. Imaging 34, 1933–1942 (2007).
Weckesser, M. et al. O-(2-[18F]fluorethyl)-L-tyrosine PET in the clinical evaluation of primary brain tumours. Eur. J. Nucl. Med. Mol. Imaging 32, 422–429 (2005).
Moulin-Romsee, G. et al. Non-invasive grading of brain tumours using dynamic amino acid PET imaging: does it work for 11C-methionine? Eur. J. Nucl. Med. Mol. Imaging 34, 2082–2087 (2007).
Cicone, F. et al. Volumetric assessment of recurrent or progressive gliomas: comparison between F-DOPA PET and perfusion-weighted MRI. Eur. J. Nucl. Med. Mol. Imaging 42, 905–915 (2015).
Galldiks, N. & Langen, K. J. Applications of PET imaging of neurological tumors with radiolabeled amino acids. Q. J. Nucl. Med. Mol. Imaging 59, 70–82 (2015).
Dunet, V., Rossier, C., Buck, A., Stupp, R. & Prior, J. O. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and metaanalysis. J. Nucl. Med. 53, 207–214 (2012).
Pichler, R. et al. Is there a place for FET PET in the initial evaluation of brain lesions with unknown significance? Eur. J. Nucl. Med. Mol. Imaging 37, 1521–1528 (2010).
Floeth, F. W. et al. 18F-FET PET differentiation of ring-enhancing brain lesions. J. Nucl. Med. 47, 776–782 (2006).
Salber, D. et al. Differential uptake of O-(2-18F-fluoroethyl)-L-tyrosine, l-3H-methionine, and 3H-deoxyglucose in brain abscesses. J. Nucl. Med. 48, 2056–2062 (2007).
Hutterer, M. et al. [18F]-fluoro-ethyl-L-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro Oncol. 15, 341–351 (2013).
Sala, Q. et al. 18F-DOPA, a clinically available PET tracer to study brain inflammation? Clin. Nucl. Med. 39, e283–e285 (2014).
Hutterer, M. et al. Epileptic activity increases cerebral amino acid transport assessed by 18F-fluoroethyl-L-tyrosine amino acid PET — a potential brain tumor mimic. J. Nucl. Med. 58, 129–137 (2017).
Smits, A. & Baumert, B. G. The clinical value of PET with amino acid tracers for gliomas WHO grade II. Int. J. Mol. Imaging 2011, 372509 (2011).
Dunet, V., Pomoni, A., Hottinger, A., Nicod-Lalonde, M. & Prior, J. O. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro Oncol. 18, 426–434 (2016).
Jansen, E. P., Dewit, L. G., van Herk, M. & Bartelink, H. Target volumes in radiotherapy for high-grade malignant glioma of the brain. Radiother. Oncol. 56, 151–156 (2000).
Aronen, H. J. et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191, 41–51 (1994).
Patel, P. et al. MR perfusion-weighted imaging in the evaluation of high-grade gliomas after treatment: a systematic review and meta-analysis. Neuro Oncol. 19, 118–127 (2016).
Wang, S. et al. Differentiating tumor progression from pseudoprogression in patients with glioblastomas using diffusion tensor imaging and dynamic susceptibility contrast MRI. AJNR Am. J. Neuroradiol. 37, 28–36 (2016).
Blasel, S. et al. Perfusion MRI in the evaluation of suspected glioblastoma recurrence. J. Neuroimaging 26, 116–123 (2016).
Stadlbauer, A. et al. Metabolic imaging of cerebral gliomas: spatial correlation of changes in O-(2-18F-fluoroethyl)-L-tyrosine PET and proton magnetic resonance spectroscopic imaging. J. Nucl. Med. 49, 721–729 (2008).
Choi, C. et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 18, 624–629 (2012). This article decribes noninvasive imaging of a genetic mutation in brain tumours by magnetic resonance spectroscopy, which is of high prognostic value.
Horská, A. & Barker, P. B. Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin. N. Am. 20, 293–310 (2010).
Hattingen, E. et al. 1H MR spectroscopic imaging with short and long echo time to discriminate glycine in glial tumours. MAGMA 22, 33–41 (2009).
Lehnhardt, F. G., Bock, C., Rohn, G., Ernestus, R. I. & Hoehn, M. Metabolic differences between primary and recurrent human brain tumors: a 1H NMR spectroscopic investigation. NMR Biomed. 18, 371–382 (2005).
Svolos, P. et al. The role of diffusion and perfusion weighted imaging in the differential diagnosis of cerebral tumors: a review and future perspectives. Cancer Imaging 14, 20 (2014).
Floeth, F. W. et al. Multimodal metabolic imaging of cerebral gliomas: positron emission tomography with [18F]fluoroethyl-L-tyrosine and magnetic resonance spectroscopy. J. Neurosurg. 102, 318–327 (2005).
Pauleit, D. et al. Comparison of 18F-FET and 18F-FDG PET in brain tumors. Nucl. Med. Biol. 36, 779–787 (2009).
Pirotte, B. et al. Combined use of 18F-fluorodeoxyglucose and 11C-methionine in 45 positron emission tomography-guided stereotactic brain biopsies. J. Neurosurg. 101, 476–483 (2004).
Plotkin, M. et al. Comparison of F-18 FET-PET with F-18 FDG-PET for biopsy planning of non-contrast-enhancing gliomas. Eur. Radiol. 20, 2496–2502 (2010).
Galldiks, N. et al. Role of O-(2-18F-fluoroethyl)-L-tyrosine PET as a diagnostic tool for detection of malignant progression in patients with low-grade glioma. J. Nucl. Med. 54, 2046–2054 (2013).
Jansen, N. L. et al. Dynamic 18F-FET PET in newly diagnosed astrocytic low-grade glioma identifies high-risk patients. J. Nucl. Med. 55, 198–203 (2014).
Kunz, M. et al. Hot spots in dynamic 18FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 13, 307–316 (2011). This article describes how the analysis of time–activity curves of the uptake of the amino acid 2-18F-fluoroethyl)- L -tyrosine with PET can detect areas with high malignancy in heterogeneous gliomas.
Jansen, N. L. et al. Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J. Nucl. Med. 56, 9–15 (2015).
Thon, N. et al. Dynamic 18F-FET PET in suspected WHO grade II gliomas defines distinct biological subgroups with different clinical courses. Int. J. Cancer 136, 2132–2145 (2015).
Unterrainer, M. et al. Serial 18F-FET PET imaging of primarily 18F-FET-negative glioma — does it make sense? J. Nucl. Med. 57, 1177–1182 (2016).
Wagner, M. et al. Heterogeneity in malignant gliomas: a magnetic resonance analysis of spatial distribution of metabolite changes and regional blood volume. J. Neurooncol. 103, 663–672 (2011).
Filss, C. P. et al. Comparison of 18F-FET PET and perfusion-weighted MR imaging: a PET/MR imaging hybrid study in patients with brain tumors. J. Nucl. Med. 55, 540–545 (2014). This study demonstrates the differences in brain tumour imaging with amino acid PET and perfusion-weighted MRI.
Widhalm, G. et al. Value of H-1-magnetic resonance spectroscopy chemical shift imaging for detection of anaplastic foci in diffusely infiltrating gliomas with non-significant contrast-enhancement. J. Neurol. Neurosurg. Psychiatry 82, 512–520 (2011).
Price, S. J. et al. Correlation of MR relative cerebral blood volume measurements with cellular density and proliferation in high-grade gliomas: an image-guided biopsy study. AJNR Am. J. Neuroradiol. 32, 501–506 (2011). This study analysis the relationship between relative cerebral blood volume mapping and tumour extent of high-grade gliomas.
Blasel, S. et al. Stripe-like increase of rCBV beyond the visible border of glioblastomas: site of tumor infiltration growing after neurosurgery. J. Neurooncol. 103, 575–584 (2011).
Sadeghi, N. et al. Apparent diffusion coefficient and cerebral blood volume in brain gliomas: relation to tumor cell density and tumor microvessel density based on stereotactic biopsies. AJNR Am. J. Neuroradiol. 29, 476–482 (2008).
Stadlbauer, A., Buchfelder, M., Doelken, M. T., Hammen, T. & Ganslandt, O. Magnetic resonance spectroscopic imaging for visualization of the infiltration zone of glioma. Cent. Eur. Neurosurg. 72, 63–69 (2011).
Kracht, L. W. et al. Delineation of brain tumor extent with [11C]l-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin. Cancer Res. 10, 7163–7170 (2004).
Lopez, W. O. et al. Correlation of 18F-fluoroethyl tyrosine positron-emission tomography uptake values and histomorphological findings by stereotactic serial biopsy in newly diagnosed brain tumors using a refined software tool. Onco Targets Ther. 8, 3803–3815 (2015).
Mosskin, M. et al. Positron emission tomography compared with magnetic resonance imaging and computed tomography in supratentorial gliomas using multiple stereotactic biopsies as reference. Acta Radiol. 30, 225–232 (1989).
Pauleit, D. et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 128, 678–687 (2005). This study demonstrated how imaging of tumour extent of gliomas is improved by amino acid PET compared with conventional MRI.
Henriksen, O. M. et al. Simultaneous evaluation of brain tumour metabolism, structure and blood volume using [18F]-fluoroethyltyrosine (FET) PET/MRI: feasibility, agreement and initial experience. Eur. J. Nucl. Med. Mol. Imaging 43, 103–112 (2016).
Mauler, J. et al. Congruency of tumour volume delineated by FET PET and MRSI. EJNMMI Phys. 2 (Suppl. 1), A61 (2015).
Rose, S. et al. Correlation of MRI-derived apparent diffusion coefficients in newly diagnosed gliomas with [18F]-fluoro-L-dopa PET: what are we really measuring with minimum ADC? AJNR Am. J. Neuroradiol. 34, 758–764 (2013).
Chen, W. Clinical applications of PET in brain tumors. J. Nucl. Med. 48, 1468–1481 (2007).
Kim, S. et al. 11C-methionine PET as a prognostic marker in patients with glioma: comparison with 18F-FDG PET. Eur. J. Nucl. Med. Mol. Imaging 32, 52–59 (2005).
Dunet, V. & Prior, J. O. Response to: performance of 18F-FET-PET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: inherent bias in meta-analysis not revealed by quality metrics. Neuro Oncol. 18, 1029–1030 (2016).
Manabe, O. et al. Oligodendroglial component complicates the prediction of tumour grading with metabolic imaging. Eur. J. Nucl. Med. Mol. Imaging 42, 896–904 (2015).
Pöpperl, G. et al. Analysis of 18F-FET PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods? J. Nucl. Med. 47, 393–403 (2006).
Albert, N. L. et al. Early static 18F-FET-PET scans have a higher accuracy for glioma grading than the standard 20–40 min scans. Eur. J. Nucl. Med. Mol. Imaging 43, 1105–1114 (2016).
Law, M. et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am. J. Neuroradiol. 24, 1989–1998 (2003).
Arvinda, H. R. et al. Glioma grading: sensitivity, specificity, positive and negative predictive values of diffusion and perfusion imaging. J. Neurooncol. 94, 87–96 (2009).
Hattingen, E. et al. 1H MRSI and progression-free survival in patients with WHO grades II and III gliomas. Neurol. Res. 32, 593–602 (2010).
Hattingen, E. et al. Prognostic value of choline and creatine in WHO grade II gliomas. Neuroradiology 50, 759–767 (2008).
Toyooka, M. et al. Tissue characterization of glioma by proton magnetic resonance spectroscopy and perfusion-weighted magnetic resonance imaging: glioma grading and histological correlation. Clin. Imaging 32, 251–258 (2008).
Hilario, A. et al. The added value of apparent diffusion coefficient to cerebral blood volume in the preoperative grading of diffuse gliomas. AJNR Am. J. Neuroradiol. 33, 701–707 (2012).
Fayed, N., Davila, J., Medrano, J. & Olmos, S. Malignancy assessment of brain tumours with magnetic resonance spectroscopy and dynamic susceptibility contrast MRI. Eur. J. Radiol. 67, 427–433 (2008).
Leclerc, X., Huisman, T. A. & Sorensen, A. G. The potential of proton magnetic resonance spectroscopy (1H-MRS) in the diagnosis and management of patients with brain tumors. Curr. Opin. Oncol. 14, 292–298 (2002).
Kim, J. H. et al. 3T 1H-MR spectroscopy in grading of cerebral gliomas: comparison of short and intermediate echo time sequences. AJNR Am. J. Neuroradiol. 27, 1412–1418 (2006).
Castillo, M., Smith, J. K., Kwock, L. & Wilber, K. Apparent diffusion coefficients in the evaluation of high-grade cerebral gliomas. AJNR Am. J. Neuroradiol. 22, 60–64 (2001).
Lev, M. H. et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. AJNR Am. J. Neuroradiol. 25, 214–221 (2004).
Wang, Q. et al. The diagnostic performance of magnetic resonance spectroscopy in differentiating high-from low-grade gliomas: a systematic review and meta-analysis. Eur. Radiol. 26, 2670–2684 (2016).
Senft, C. et al. Diagnostic value of proton magnetic resonance spectroscopy in the noninvasive grading of solid gliomas: comparison of maximum and mean choline values. Neurosurgery 65, 908–913 (2009).
Guzman-De-Villoria, J. A., Mateos-Perez, J. M., Fernandez-Garcia, P., Castro, E. & Desco, M. Added value of advanced over conventional magnetic resonance imaging in grading gliomas and other primary brain tumors. Cancer Imaging 14, 35 (2014).
Usinskiene, J. et al. Optimal differentiation of high- and low-grade glioma and metastasis: a meta-analysis of perfusion, diffusion, and spectroscopy metrics. Neuroradiology 58, 339–350 (2016). This meta-analysis gives an overview of the role of advanced MRI methods for tumour grading.
Galldiks, N. et al. Volumetry of [11C]-methionine positron emission tomographic uptake as a prognostic marker before treatment of patients with malignant glioma. Mol. Imaging 11, 516–527 (2012).
Piroth, M. D. et al. Prognostic value of early [18F]fluoroethyltyrosine positron emission tomography after radiochemotherapy in glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 80, 176–184 (2011).
Suchorska, B. et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 84, 710–719 (2015).
Villani, V. et al. The role of PET [18F]FDOPA in evaluating low-grade glioma. Anticancer Res. 35, 5117–5122 (2015).
Floeth, F. W. et al. Prognostic value of O-(2-18F-fluoroethyl)-L-tyrosine PET and MRI in low-grade glioma. J. Nucl. Med. 48, 519–527 (2007).
Jansen, N. L. et al. MRI-suspected low-grade glioma: is there a need to perform dynamic FET PET? Eur. J. Nucl. Med. Mol. Imaging 39, 1021–1029 (2012).
Hirai, T. et al. Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am. J. Neuroradiol. 29, 1505–1510 (2008).
Jain, R. et al. Genomic mapping and survival prediction in glioblastoma: molecular subclassification strengthened by hemodynamic imaging biomarkers. Radiology 267, 212–220 (2013).
Shiroishi, M. S., Boxerman, J. L. & Pope, W. B. Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma. Neuro Oncol. 18, 467–478 (2016).
Law, M. et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247, 490–498 (2008).
Nakamura, H., Murakami, R., Hirai, T., Kitajima, M. & Yamashita, Y. Can MRI-derived factors predict the survival in glioblastoma patients treated with postoperative chemoradiation therapy? Acta Radiol. 54, 214–220 (2013).
Saraswathy, S. et al. Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J. Neurooncol. 91, 69–81 (2009).
Ellingson, B. M. et al. Pretreatment ADC histogram analysis is a predictive imaging biomarker for bevacizumab treatment but not chemotherapy in recurrent glioblastoma. AJNR Am. J. Neuroradiol. 35, 673–679 (2014).
Brandsma, D. & van den Bent, M. J. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr. Opin. Neurol. 22, 633–638 (2009).
Wen, P. Y. et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 28, 1963–1972 (2010). This study describes the limitations of conventional MRI in response assessment of high-grade gliomas.
Kebir, S. et al. Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin. Cancer Res. 22, 2190–2196 (2016).
Galldiks, N. et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 42, 685–695 (2015).
Karunanithi, S. et al. 18F-FDOPA PET/CT for detection of recurrence in patients with glioma: prospective comparison with 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 40, 1025–1035 (2013).
Rachinger, W. et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 57, 505–511 (2005).
Minamimoto, R. et al. Differentiation of brain tumor recurrence from post-radiotherapy necrosis with 11C-methionine PET: visual assessment versus quantitative assessment. PLoS ONE 10, e0132515 (2015).
Nihashi, T., Dahabreh, I. J. & Terasawa, T. Diagnostic accuracy of PET for recurrent glioma diagnosis: a meta-analysis. AJNR Am. J. Neuroradiol. 34, 944–950 (2013).
Ceccon, G. et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro Oncol. 19, 281–288 (2016). This article reports on the diagnostic accuracy of O -(2-18F-fluoroethyl)- L -tyrosine PET for the differentiation of disease relapse from radiation injury in patients with brain metastasis.
Galldiks, N. et al. Role of O-(2-18F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J. Nucl. Med. 53, 1367–1374 (2012).
Lizarraga, K. J. et al. 18F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J. Nucl. Med. 55, 30–36 (2014).
Terakawa, Y. et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J. Nucl. Med. 49, 694–699 (2008).
Tsuyuguchi, N. et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J. Neurosurg. 98, 1056–1064 (2003).
Hodi, F. S. et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol. 34, 1510–1517 (2016).
Kebir, S. et al. Dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET imaging for the detection of checkpoint inhibitor-related pseudoprogression in melanoma brain metastases. Neuro Oncol. 18, 1462–1464 (2016). This report shows the potential of amino acid PET to detect checkpoint inhibitor-related pseudoprogression in brain metastases.
Choi, Y. J., Kim, H. S., Jahng, G. H., Kim, S. J. & Suh, D. C. Pseudoprogression in patients with glioblastoma: added value of arterial spin labeling to dynamic susceptibility contrast perfusion MR imaging. Acta Radiol. 54, 448–454 (2013).
Hu, L. S. et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am. J. Neuroradiol. 30, 552–558 (2009).
Kreis, R. Issues of spectral quality in clinical 1H-magnetic resonance spectroscopy and a gallery of artifacts. NMR Biomed. 17, 361–381 (2004).
Hygino da Cruz, L. C. Jr, Rodriguez, I., Domingues, R. C., Gasparetto, E. L. & Sorensen, A. G. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am. J. Neuroradiol. 32, 1978–1985 (2011).
Zhang, H. et al. Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: a systematic review and meta-analysis. Eur. J. Radiol. 83, 2181–2189 (2014).
Galldiks, N. et al. Assessment of treatment response in patients with glioblastoma using [18F]fluoroethyl-L-tyrosine PET in comparison to MRI. J. Nucl. Med. 53, 1048–1057 (2012).
Galldiks, N. et al. Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging 33, 516–524 (2006).
Galldiks, N. et al. Patient-tailored, imaging-guided, long-term temozolomide chemotherapy in patients with glioblastoma. Mol. Imaging 9, 40–46 (2010).
Pöpperl, G. et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 32, 1018–1025 (2005).
Popperl, G. et al. Serial O-(2-[18F]fluoroethyl)-L-tyrosine PET for monitoring the effects of intracavitary radioimmunotherapy in patients with malignant glioma. Eur. J. Nucl. Med. Mol. Imaging 33, 792–800 (2006).
Galldiks, N. et al. Earlier diagnosis of progressive disease during bevacizumab treatment using O-(2-18F-fluorethyl)-L-tyrosine positron emission tomography in comparison with magnetic resonance imaging. Mol. Imaging 12, 273–276 (2013).
Hutterer, M. et al. O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J. Nucl. Med. 52, 856–864 (2011).
Schwarzenberg, J. et al. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin. Cancer Res. 20, 3550–3559 (2014).
Moffat, B. A. et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc. Natl Acad. Sci. USA 102, 5524–5529 (2005).
Pope, W. B. et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J. Neurooncol. 108, 491–498 (2012).
Rahman, R. et al. Histogram analysis of apparent diffusion coefficient within enhancing and nonenhancing tumor volumes in recurrent glioblastoma patients treated with bevacizumab. J. Neurooncol. 119, 149–158 (2014).
Schmainda, K. M. et al. Dynamic-susceptibility contrast agent MRI measures of relative cerebral blood volume predict response to bevacizumab in recurrent high-grade glioma. Neuro Oncol. 16, 880–888 (2014).
Gizewski, E. R., Monninghoff, C. & Forsting, M. Perspectives of ultra-high-field MRI in neuroradiology. Clin. Neuroradiol. 25 (Suppl. 2), 267–273 (2015).
Ren, J., Sherry, A. D. & Malloy, C. R. 31P-MRS of healthy human brain: ATP synthesis, metabolite concentrations, pH, and T1 relaxation times. NMR Biomed. 28, 1455–1462 (2015).
Ward, K. M., Aletras, A. H. & Balaban, R. S. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J. Magn. Reson. 143, 79–87 (2000).
Walker-Samuel, S. et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat. Med. 19, 1067–1072 (2013).
Sagiyama, K. et al. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc. Natl Acad. Sci. USA 111, 4542–4547 (2014).
Pellegatta, S. et al. Effective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial glioma. Acta Neuropathol. Commun. 3, 4 (2015).
Andronesi, O. C. et al. Treatment response assessment in IDH-mutant glioma patients by noninvasive 3D functional spectroscopic mapping of 2-hydroxyglutarate. Clin. Cancer Res. 22, 1632–1641 (2016).
Winkeler, A. et al. The translocator protein ligand [18F]DPA-714 images glioma and activated microglia in vivo. Eur. J. Nucl. Med. Mol. Imaging 39, 811–823 (2012).
Roncaroli, F., Su, Z., Herholz, K., Gerhard, A. & Turkheimer, F. E. TSPO expression in brain tumours: is TSPO a target for brain tumour imaging? Clin. Transl Imaging 4, 145–156 (2016).
Su, Z. et al. The 18-kDa mitochondrial translocator protein in human gliomas: an 11C-(R)PK11195 PET imaging and neuropathology study. J. Nucl. Med. 56, 512–517 (2015).
Jensen, P. et al. TSPO imaging in glioblastoma multiforme: a direct comparison between 123I-CLINDE SPECT, 18F-FET PET, and gadolinium-enhanced MR imaging. J. Nucl. Med. 56, 1386–1390 (2015).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, provided substantial contributions to discussion of content, wrote the article and reviewed and edited the manuscript before submission. K.-J.L. and N.G. contributed equally to the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Adjuvant chemotherapy
-
Administration of chemotherapy after surgery or radiation treatment for cancer to target remaining malignant cells.
- T1-weighted and T2-weighted sequences
-
Standard morphological images weighted by the longitudinal (T1) and transverse (T2) relaxation times of the protons.
- Perfusion-weighted imaging
-
Image acquisition techniques that highlight fluids moving through arteries, veins and capillaries.
- Diffusion-weighted imaging
-
Imaging technique designed to weight the MRI signal by the amount of diffusion (random thermal motion) of water molecules.
- Fluid-attenuated inversion recovery
-
MRI technique that uses inversion recovery, in which the signal from water is reduced by timing the delay of the inversion pulse.
- Cerebral blood flow
-
Flow of capillary blood per unit mass through the brain tissue (units: ml/min/100 g).
- Relative cerebral blood volume
-
The volume of blood in a brain lesion in relation to the normal brain tissue.
- Mean transit time
-
The average time, in seconds, that red blood cells spend within a given volume of capillary circulation.
- Single voxel spectroscopy
-
A magnetic resonance spectroscopy technique used to assess the concentration of metabolites in a defined region of interest.
- Brownian motion
-
Continuous random movement of particles suspended in a fluid, which arises from collisions with the fluid molecules.
- Fractional anisotropy
-
Fractional anisotropy (FA) is a scalar parameter (0 ≤ FA ≤1) used to quantify the degree of anisotropy of the diffusion process.
- Local maxima
-
Area with maximum signal of different parameters in the tumour area
- Hotspots
-
Area with locally increased signal of different parameters in the corresponding image.
- Pseudoprogression
-
A phenomenon whereby tumours initially 'grow' on MRI due to immune infiltrate, but then decrease in size.
- Convection-enhanced delivery
-
A therapeutic strategy to facilitate delivery of pharmaceuticals. Placement of small-diameter catheters directly into the brain tumour is followed by infusion of therapeutics.
- Chemical exchange saturation transfer
-
An MRI technique in which exogenous or endogenous compounds containing exchangeable molecules are selectively saturated and, after transfer of this saturation, detected indirectly through the water signal with enhanced sensitivity.
Rights and permissions
About this article
Cite this article
Langen, KJ., Galldiks, N., Hattingen, E. et al. Advances in neuro-oncology imaging. Nat Rev Neurol 13, 279–289 (2017). https://doi.org/10.1038/nrneurol.2017.44
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.44
This article is cited by
-
Assessment of Brain Tumour Perfusion Using Early-Phase 18F-FET PET: Comparison with Perfusion-Weighted MRI
Molecular Imaging and Biology (2024)
-
Advances in PET imaging of cancer
Nature Reviews Cancer (2023)
-
Multiparametric Analysis Combining DSC-MR Perfusion and [18F]FET-PET is Superior to a Single Parameter Approach for Differentiation of Progressive Glioma from Radiation Necrosis
Clinical Neuroradiology (2023)
-
How Much is Enough? Impact of Efflux Transporters on Drug delivery Leading to Efficacy in the Treatment of Brain Tumors
Pharmaceutical Research (2023)
-
[18F]FET-PET in children and adolescents with central nervous system tumors: does it support difficult clinical decision-making?
European Journal of Nuclear Medicine and Molecular Imaging (2023)