Key Points
-
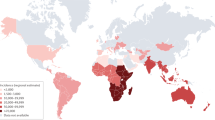
Tuberculous meningitis (TBM) causes death and disability, with especially high rates of poor outcomes in children and individuals with an HIV-1 co-infection
-
Important risk factors for poor outcome are delayed diagnosis, delayed treatment, advanced disease, and antitubercular drug resistance
-
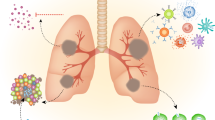
Intracerebral and spinal pathology in TBM is mediated by a dysregulated inflammatory response that contributes to meningitis, tuberculoma formation, arteritis, obstruction of cerebrospinal fluid (CSF) flow, and vascular complications including stroke
-
Diagnosis of TBM is insensitive and laborious; clinical scoring algorithms are imperfect and few rigorous evaluations of diagnostics have been performed
-
Multidrug antitubercular antibiotic therapy is the mainstay of treatment; however, CSF penetration is probably a major limitation of these therapies, and evidence supporting dosage and treatment combinations is weak
-
The supportive management of TBM complications, which include hyponatraemia, hydrocephalus, hypoxic brain damage and infarction, is poorly understood and researched, but is vital to outcome
Abstract
Tuberculosis remains a global health problem, with an estimated 10.4 million cases and 1.8 million deaths resulting from the disease in 2015. The most lethal and disabling form of tuberculosis is tuberculous meningitis (TBM), for which more than 100,000 new cases are estimated to occur per year. In patients who are co-infected with HIV-1, TBM has a mortality approaching 50%. Study of TBM pathogenesis is hampered by a lack of experimental models that recapitulate all the features of the human disease. Diagnosis of TBM is often delayed by the insensitive and lengthy culture technique required for disease confirmation. Antibiotic regimens for TBM are based on those used to treat pulmonary tuberculosis, which probably results in suboptimal drug levels in the cerebrospinal fluid, owing to poor blood–brain barrier penetrance. The role of adjunctive anti-inflammatory, host-directed therapies — including corticosteroids, aspirin and thalidomide — has not been extensively explored. To address this deficit, two expert meetings were held in 2009 and 2015 to share findings and define research priorities. This Review summarizes historical and current research into TBM and identifies important gaps in our knowledge. We will discuss advances in the understanding of inflammation in TBM and its potential modulation; vascular and hypoxia-mediated tissue injury; the role of intensified antibiotic treatment; and the importance of rapid and accurate diagnostics and supportive care in TBM.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ducomble, T. et al. The burden of extrapulmonary and meningitis tuberculosis: an investigation of national surveillance data, Germany, 2002 to 2009. Euro Surveill. 18, 20436 (2013).
Pan, Y. et al. Host and microbial predictors of childhood extrathoracic tuberculosis and tuberculosis meningitis. Pediatr. Infect. Dis. J. 34, 1289–1295 (2015).
Trunz, B. B., Fine, P. & Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367, 1173–1180 (2006).
World Health Organization. Global Tuberculosis Report 21st edition (WHO, 2016).
Gomes, T. et al. Epidemiology of extrapulmonary tuberculosis in Brazil: a hierarchical model. BMC Infect. Dis. 14, 9 (2014).
Chiang, S. S. et al. Treatment outcomes of childhood tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect. Dis. 14, 947–957 (2014).
Marais, S., Pepper, D. J., Schutz, C., Wilkinson, R. J. & Meintjes, G. Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS ONE 6, e20077 (2011).
Rana, F. S. et al. Autopsy study of HIV-1-positive and HIV-1-negative adult medical patients in Nairobi. Kenya. J. Acquir. Immune Def. Syndr. 24, 23–29 (2000).
Thwaites, G. E. et al. The influence of HIV infection on clinical presentation, response to treatment, and outcome in adults with Tuberculous meningitis. J. Infect. Dis. 192, 2134–2141 (2005).
Heemskerk, A. D. et al. Intensified antituberculosis therapy in adults with tuberculous meningitis. N. Engl. J. Med. 374, 124–134 (2016).
Tho, D. Q. et al. Influence of antituberculosis drug resistance and Mycobacterium tuberculosis lineage on outcome in HIV-associated tuberculous meningitis. Antimicrob. Agents Chemother. 56, 3074–3079 (2012).
Rich, A. R. The Pathogenesis of Tuberculosis (C. C. Thomas, 1946).
Donald, P. R. & Schoeman, J. F. Tuberculous meningitis. N. Engl. J. Med. 351, 1719–1720 (2004).
Tucker, E. W. et al. Microglia activation in a pediatric rabbit model of tuberculous meningitis. Dis. Model. Mech. 9, 1497–1506 (2016).
van Leeuwen, L. M. et al. Modeling tuberculous meningitis in zebrafish using Mycobacterium marinum. Dis. Model. Mech. 7, 1111–1122 (2014).
Donald, P. R., Schaaf, H. S. & Schoeman, J. F. Tuberculous meningitis and miliary tuberculosis: the Rich focus revisited. J. Infect. 50, 193–195 (2005).
Berenguer, J. et al. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 326, 668–672 (1992).
Shafer, R. W., Goldberg, R., Sierra, M. & Glatt, A. E. Frequency of Mycobacterium tuberculosis bacteremia in patients with tuberculosis in an area endemic for AIDS. Am. Rev. Respir. Dis. 140, 1611–1613 (1989).
Marais, S., Pepper, D. J., Marais, B. J. & Torok, M. E. HIV-associated tuberculous meningitis — diagnostic and therapeutic challenges. Tuberculosis 90, 367–374 (2010).
Thwaites, G. E. et al. Effect of antituberculosis drug resistance on response to treatment and outcome in adults with tuberculous meningitis. J. Infect. Dis. 192, 79–88 (2005).
Pepper, D. J. et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin. Infect. Dis. 48, e96–e107 (2009).
Burn, C. G. & Finley, K. H. The role of hypersensitivity in the production of experimental meningitis: I. Experimental meningitis in tuberculous animals. J. Exp. Med. 56, 203–221 (1932).
Peterson, P. K. et al. CD14 receptor-mediated uptake of nonopsonized Mycobacterium tuberculosis by human microglia. Infect. Immun. 63, 1598–1602 (1995).
Lee, H. M., Kang, J., Lee, S. J. & Jo, E. K. Microglial activation of the NLRP3 inflammasome by the priming signals derived from macrophages infected with mycobacteria. Glia 61, 441–452 (2013).
Ray, G., Aneja, S., Jain, M. & Batra, S. Evaluation of free radical status in CSF in childhood meningitis. Ann. Trop. Paediatr. 20, 115–120 (2000).
Mason, S. et al. A hypothetical astrocyte–microglia lactate shuttle derived from a 1H NMR metabolomics analysis of cerebrospinal fluid from a cohort of South African children with tuberculous meningitis. Metabolomics 11, 822–837 (2015).
Thwaites, G. E. et al. Pathophysiology and prognosis in vietnamese adults with tuberculous meningitis. J. Infect. Dis. 188, 1105–1115 (2003).
Misra, U. K., Kalita, J., Bhoi, S. K. & Singh, R. K. A study of hyponatremia in tuberculous meningitis. J. Neurol. Sci. 367, 152–157 (2016).
Dhanwal, D. K., Vyas, A., Sharma, A. & Saxena, A. Hypothalamic pituitary abnormalities in tubercular meningitis at the time of diagnosis. Pituitary 13, 304–310 (2010).
Rock, R. B. et al. Mycobacterium tuberculosis-induced cytokine and chemokine expression by human microglia and astrocytes: effects of dexamethasone. J. Infect. Dis. 192, 2054–2058 (2005).
Simmons, C. P. et al. The clinical benefit of adjunctive dexamethasone in tuberculous meningitis is not associated with measurable attenuation of peripheral or local immune responses. J. Immunol. 175, 579–590 (2005).
Simmons, C. P. et al. Pretreatment intracerebral and peripheral blood immune responses in vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J. Immunol. 176, 2007–2014 (2006).
Tsenova, L., Sokol, K., Freedman, V. H. & Kaplan, G. A combination of thalidomide plus antibiotics protects rabbits from mycobacterial meningitis-associated death. J. Infect. Dis. 177, 1563–1572 (1998).
Tsenova, L., Bergtold, A., Freedman, V. H., Young, R. A. & Kaplan, G. Tumor necrosis factor α is a determinant of pathogenesis and disease progression in mycobacterial infection in the central nervous system. Proc. Natl Acad. Sci. USA 96, 5657–5662 (1999).
Akalin, H., Akdis, A. C., Mistik, R., Helvaci, S. & Kilicturgay, K. Cerebrospinal fluid interleukin-1ß/interleukin-1 receptor antagonist balance and tumor necrosis factor-α concentrations in tuberculous, viral and acute bacterial meningitis. Scand. J. Infect. Dis. 26, 667–674 (1994).
Donald, P. R. et al. Concentrations of interferon γ, tumor necrosis factor α, and interleukin-1ß in the cerebrospinal fluid of children treated for tuberculous meningitis. Clin. Infect. Dis. 21, 924–929 (1995).
Schoeman, J. F. et al. Adjunctive thalidomide therapy for childhood tuberculous meningitis: results of a randomized study. J. Child Neurol. 19, 250–257 (2004).
Yadav, A. et al. Correlation of CSF proinflammatory cytokines with MRI in tuberculous meningitis. Acad. Radiol. 17, 194–200 (2010).
Misra, U. K. et al. A study of cytokines in tuberculous meningitis: clinical and MRI correlation. Neurosci. Lett. 483, 6–10 (2010).
Marais, S. et al. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin. Infect. Dis. 56, 450–460 (2013).
Marais, S. et al. Neutrophil-associated central nervous system inflammation in tuberculous meningitis immune reconstitution inflammatory syndrome. Clin. Infect. Dis. 59, 1638–1647 (2014).
Marais, S. et al. Inflammasome activation underlies central nervous system deterioration in HIV-associated tuberculosis. J. Infect. Dis. 215, 677–686 (2016).
Kalita, J., Prasad, S. & Misra, U. K. Predictors of paradoxical tuberculoma in tuberculous meningitis. Int. J. Tuberc. Lung Dis. 18, 486–491 (2014).
Thwaites, G. E. et al. Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: an observational study. Lancet Neurol. 6, 230–236 (2007).
Thomas, M. M. et al. Rapid diagnosis of Mycobacterium tuberculosis meningitis by enumeration of cerebrospinal fluid antigen-specific T-cells. Int. J. Tuberc. Lung Dis. 12, 651–657 (2008).
Caccamo, N. et al. Phenotypical and functional analysis of memory and effector human CD8 T cells specific for mycobacterial antigens. J. Immunol. 177, 1780–1785 (2006).
Dieli, F. et al. Predominance of Vγ9/Vδ2 T lymphocytes in the cerebrospinal fluid of children with tuberculous meningitis: reversal after chemotherapy. Mol. Med. 5, 301–312 (1999).
Mansour, A. M. et al. Relationship between intracranial granulomas and cerebrospinal fluid levels of gamma interferon and interleukin-10 in patients with tuberculous meningitis. Clin. Diagn. Lab Immunol. 12, 363–365 (2005).
Yang, Q. et al. IP-10 and MIG are compartmentalized at the site of disease during pleural and meningeal tuberculosis and are decreased after antituberculosis treatment. Clin. Vaccine Immunol. 21, 1635–1644 (2014).
Green, J. A. et al. Dexamethasone, cerebrospinal fluid matrix metalloproteinase concentrations and clinical outcomes in tuberculous meningitis. PLoS ONE 4, e7277 (2009).
Green, J. A. et al. Mycobacterium tuberculosis-infected human monocytes down-regulate microglial MMP-2 secretion in CNS tuberculosis via TNFα, NFκB, p38 and caspase 8 dependent pathways. J. Neuroinflamm. 8, 46 (2011).
Visser, D. H. et al. Host immune response to tuberculous meningitis. Clin. Infect. Dis. 60, 177–187 (2015).
Matsuyama, W. et al. Expression of vascular endothelial growth factor in tuberculous meningitis. J. Neurol. Sci. 186, 75–79 (2001).
Misra, U. K., Kalita, J., Singh, A. P. & Prasad, S. Vascular endothelial growth factor in tuberculous meningitis. Int. J. Neurosci. 123, 128–132 (2013).
Ozden, M., Kalkan, A., Demirdag, K., Denk, A. & Kilic, S. S. Hepatocyte growth factor (HGF) in patients with hepatitis B and meningitis. J. Infect. 49, 229–235 (2004).
Comas, I. et al. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat. Genet. 45, 1176–1182 (2013).
Caws, M. et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 4, e1000034 (2008).
Faksri, K. et al. Epidemiological trends and clinical comparisons of Mycobacterium tuberculosis lineages in Thai TB meningitis. Tuberculosis (Edinb.) 91, 594–600 (2011).
Nicol, M. P. et al. Distribution of strain families of Mycobacterium tuberculosis causing pulmonary and extrapulmonary disease in hospitalized children in Cape Town, South Africa. J. Clin. Microbiol. 43, 5779–5781 (2005).
Wang, J. et al. DNA polymorphism of Mycobacterium tuberculosis PE_PGRS33 gene among clinical isolates of pediatric TB patients and its associations with clinical presentation. Tuberculosis (Edinb.) 91, 287–292 (2011).
Tobin, D. M. et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 148, 434–446 (2012).
Thuong, N. et al. Leukotriene A4 hydrolase genotype and HIV infection influence intracerebral inflammation and survival from tuberculous meningitis J. Infect. Dis. 215, 1020–1028 (2017).
van Laarhoven, A. et al. Clinical parameters, routine inflammatory markers, and LTA4H genotype as predictors of mortality among 608 patients with tuberculous meningitis in Indonesia. J. Infect. Dis. 215, 1029–1039 (2017).
Thwaites, G. E., van Toorn, R. & Schoeman, J. Tuberculous meningitis: more questions, still too few answers. Lancet Neurol. 12, 999–1010 (2013).
van Well, G. T. et al. Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics 123, e1–e8 (2009).
Miftode, E. G. et al. Tuberculous meningitis in children and adults: a 10-year retrospective comparative analysis. PLoS ONE 10, e0133477 (2015).
Torok, M. E. et al. Clinical and microbiological features of HIV-associated tuberculous meningitis in Vietnamese adults. PLoS ONE 3, e1772 (2008).
Streptomycin in Tuberculosis Trials Commitee, Medical Research Council. Streptomycin treatment of tuberculous meningitis. Lancet 1, 582–596 (1948).
Dhawan, S. R. et al. Predictors of neurological outcome of tuberculous meningitis in childhood: A prospective cohort study from a developing country. J. Child Neurol. 31, 1622–1627 (2016).
Brancusi, F., Farrar, J. & Heemskerk, D. Tuberculous meningitis in adults: a review of a decade of developments focusing on prognostic factors for outcome. Future Microbiol. 7, 1101–1116 (2012).
Vinnard, C. et al. The long-term mortality of tuberculosis meningitis patients in new york city: a cohort study. Clin. Infect. Dis. 64, 401–407 (2016).
Thwaites, G. E. et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 351, 1741–1751 (2004).
Garg, R. K., Malhotra, H. S. & Jain, A. Neuroimaging in tuberculous meningitis. Neurol. India 64, 219–227 (2016).
van der Merwe, D. J., Andronikou, S., Van Toorn, R. & Pienaar, M. Brainstem ischemic lesions on MRI in children with tuberculous meningitis: with diffusion weighted confirmation. Childs Nerv. Syst. 25, 949–954 (2009).
Omar, N., Andronikou, S., van Toorn, R. & Pienaar, M. Diffusion-weighted magnetic resonance imaging of borderzone necrosis in paediatric tuberculous meningitis. J. Med. Imag. Radiat. Oncol. 55, 563–570 (2011).
Singh, B. et al. Computed tomography angiography in patients with tuberculous meningitis. J. Infect. 64, 565–572 (2012).
Lu, T. T. et al. Magnetic resonance angiography manifestations and prognostic significance in HIV-negative tuberculosis meningitis. Int. J. Tuberc. Lung Dis. 19, 1448–1454 (2015).
Kalita, J., Prasad, S., Maurya, P. K., Kumar, S. & Misra, U. K. MR angiography in tuberculous meningitis. Acta Radiol. 53, 324–329 (2012).
Rohlwink, U. K. et al. Imaging features of the brain, cerebral vessels and spine in pediatric tuberculous meningitis with associated hydrocephalus. Pediatr. Infect. Dis. J. 35, e301–e310 (2016).
Thwaites, G. E., Chau, T. T. & Farrar, J. J. Improving the bacteriological diagnosis of tuberculous meningitis. J. Clin. Microbiol. 42, 378–379 (2004).
Stewart, S. M. The bacteriological diagnosis of tuberculous meningitis. J. Clin. Pathol. 6, 241–242 (1953).
Caws, M. et al. Evaluation of the MODS culture technique for the diagnosis of tuberculous meningitis. PLoS ONE 2, e1173 (2007).
Pai, M. et al. Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect. Dis. 3, 633–643 (2003).
Patel, V. B. et al. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med. 10, e1001536 (2013).
Bahr, N. C. et al. Improved diagnostic sensitivity for tuberculous meningitis with Xpert((R)) MTB/RIF of centrifuged CSF. Int. J. Tuberc. Lung Dis. 19, 1209–1215 (2015).
Nhu, N. T. et al. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J. Clin. Microbiol. 52, 226–233 (2014).
Boyles, T. H. & Thwaites, G. E. Appropriate use of the Xpert(R) MTB/RIF assay in suspected tuberculous meningitis. Int. J. Tuberc. Lung Dis. 19, 276–277 (2015).
Bahr, N. C. et al. GeneXpert MTB/Rif to diagnose tuberculous meningitis: perhaps the first test but not the last. Clin. Infect. Dis. 62, 1133–1135 (2016).
World Health Organization. Meeting Report of a Technical Expert Consultation: Non-inferiority Analysis of Xpert MTB/RIF Ultra Compared to Xpert MTB/RIF (WHO, 2017).
Cox, J. A. et al. Accuracy of lipoarabinomannan and Xpert MTB/RIF testing in cerebrospinal fluid to diagnose tuberculous meningitis in an autopsy cohort of HIV-infected adults. J. Clin. Microbiol. 53, 2667–2673 (2015).
Patel, V. B. et al. Utility of a novel lipoarabinomannan assay for the diagnosis of tuberculous meningitis in a resource-poor high-HIV prevalence setting. Cerebrospinal Fluid Res. 6, 13 (2009).
Patel, V. B. et al. Comparison of a clinical prediction rule and a LAM antigen-detection assay for the rapid diagnosis of TBM in a high HIV prevalence setting. PLoS ONE 5, e15664 (2010).
Yu, J., Wang, Z. J., Chen, L. H. & Li, H. H. Diagnostic accuracy of interferon-gamma release assays for tuberculous meningitis: a meta-analysis. Int. J. Tuberc. Lung Dis. 20, 494–499 (2016).
Tuon, F. F. et al. Adenosine deaminase and tuberculous meningitis — a systematic review with meta-analysis. Scand. J. Infect. Dis. 42, 198–207 (2010).
Xu, H. B., Jiang, R. H., Li, L., Sha, W. & Xiao, H. P. Diagnostic value of adenosine deaminase in cerebrospinal fluid for tuberculous meningitis: a meta-analysis. Int. J. Tuberc. Lung Dis. 14, 1382–1387 (2010).
Bullock, M. R. & Welchman, J. M. Diagnostic and prognostic features of tuberculous meningitis on CT scanning. J. Neurol. Neurosurg. Psychiatry 45, 1098–1101 (1982).
Andronikou, S., Smith, B., Hatherhill, M., Douis, H. & Wilmshurst, J. Definitive neuroradiological diagnostic features of tuberculous meningitis in children. Pediatr. Radiol. 34, 876–885 (2004).
Przybojewski, S., Andronikou, S. & Wilmshurst, J. Objective CT criteria to determine the presence of abnormal basal enhancement in children with suspected tuberculous meningitis. Pediatr. Radiol 36, 687–696 (2006).
Botha, H. et al. Reliability and diagnostic performance of CT imaging criteria in the diagnosis of tuberculous meningitis. PLoS ONE 7, e38982 (2012).
Pienaar, M., Andronikou, S. & van Toorn, R. MRI to demonstrate diagnostic features and complications of TBM not seen with CT. Childs Nerv. Syst. 25, 941–947 (2009).
Janse van Rensburg, P., Andronikou, S., van Toorn, R. & Pienaar, M. Magnetic resonance imaging of miliary tuberculosis of the central nervous system in children with tuberculous meningitis. Pediatr. Radiol 38, 1306–1313 (2008).
Kalita, J., Misra, U. K. & Nair, P. P. Predictors of stroke and its significance in the outcome of tuberculous meningitis. J. Stroke Cerebrovasc Dis. 18, 251–258 (2009).
Dekker, G. et al. MRI findings in children with tuberculous meningitis: a comparison of HIV-infected and non-infected patients. Childs Nerv. Syst. 27, 1943–1949 (2011).
Andronikou, S. et al. Value of early follow-up CT in paediatric tuberculous meningitis. Pediatr. Radiol. 35, 1092–1099 (2005).
Gambhir, S. et al. Role of 18F-FDG PET in demonstrating disease burden in patients with tuberculous meningitis. J. Neurol. Sci. 370, 196–200 (2016).
Solomons, R. S. et al. Chest radiograph findings in children with tuberculous meningitis. Int. J. Tuberc. Lung Dis. 19, 200–204 (2015).
Thwaites, G. E. et al. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet 360, 1287–1292 (2002).
Sunbul, M., Atilla, A., Esen, S., Eroglu, C. & Leblebicioglu, H. Thwaites' diagnostic scoring and the prediction of tuberculous meningitis. Med. Princ. Pract. 14, 151–154 (2005).
Torok, M. E. et al. Validation of a diagnostic algorithm for adult tuberculous meningitis. Am. J. Trop. Med. Hyg. 77, 555–559 (2007).
Vibha, D. et al. Validation of diagnostic algorithm to differentiate between tuberculous meningitis and acute bacterial meningitis. Clin. Neurol. Neurosurgery 114, 639–644 (2012).
Zhang, Y. L., Lin, S., Shao, L. Y., Zhang, W. H. & Weng, X. H. Validation of Thwaites' diagnostic scoring system for the differential diagnosis of tuberculous meningitis and bacterial meningitis. Jpn. J. Infect. Dis. 67, 428–431 (2014).
Saavedra, J. S. et al. Validation of Thwaites Index for diagnosing tuberculous meningitis in a Colombian population. J. Neurol. Sci. 370, 112–118 (2016).
Checkley, A. M., Njalale, Y., Scarborough, M. & Zjilstra, E. E. Sensitivity and specificity of an index for the diagnosis of TB meningitis in patients in an urban teaching hospital in Malawi. Trop. Med. Int. Health 13, 1042–1046 (2008).
Marais, S. et al. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect. Dis. 10, 803–812 (2010).
Solomons, R. S., Visser, D. H., Marais, B. J., Schoeman, J. F. & van Furth, A. M. Diagnostic accuracy of a uniform research case definition for TBM in children: a prospective study. Int. J. Tuberc. Lung Dis. 20, 903–908 (2016).
Donald, P. R. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis (Edinb.) 90, 279–292 (2010).
Donald, P. R. The chemotherapy of tuberculous meningitis in children and adults. Tuberculosis (Edinb.) 90, 375–392 (2010).
World Health Organization. Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children (WHO, 2014).
Thwaites, G. et al. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J. Infect. 59, 167–187 (2009).
van Toorn, R. et al. Short intensified treatment in children with drug-susceptible tuberculous meningitis. Pediatr. Infect. Dis. J. 33, 248–252 (2014).
Savic, R. M. et al. Pediatric tuberculous meningitis: model-based approach to determining optimal doses of the anti-tuberculosis drugs rifampin and levofloxacin for children. Clin. Pharmacol. Ther. 98, 622–629 (2015).
Ruslami, R. et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect. Dis. 13, 27–35 (2013).
Boeree, M. J. et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect. Dis. 17, 39–49 (2017).
Alffenaar, J. W. et al. Pharmacokinetics of moxifloxacin in cerebrospinal fluid and plasma in patients with tuberculous meningitis. Clin. Infect. Dis. 49, 1080–1082 (2009).
Thwaites, G. E. et al. Randomized pharmacokinetic and pharmacodynamic comparison of fluoroquinolones for tuberculous meningitis. Antimicrob. Agents Chemother. 55, 3244–3253 (2011).
Seddon, J. A. et al. Impact of drug resistance on clinical outcome in children with tuberculous meningitis. Pediatr. Infect. Dis. J. 31, 711–716 (2012).
Shane, S. J., Clowater, R. A. & Riley, C. The treatment of tuberculous meningitis with cortisone and streptomycin. Can. Med. Assoc. J. 67, 13–15 (1952).
Prasad, K., Singh, M. B. & Ryan, H. Corticosteroids for managing tuberculous meningitis. Cochrane Database Syst. Rev. 4, CD002244 (2016).
Singh, A. K. et al. Paradoxical reaction in tuberculous meningitis: presentation, predictors and impact on prognosis. BMC Infect. Dis. 16, 306 (2016).
Garg, R. K., Malhotra, H. S. & Kumar, N. Paradoxical reaction in HIV negative tuberculous meningitis. J. Neurol. Sci. 340, 26–36 (2014).
Schoeman, J. F., Andronikou, S., Stefan, D. C., Freeman, N. & van Toorn, R. Tuberculous meningitis-related optic neuritis: recovery of vision with thalidomide in 4 consecutive cases. J. Child Neurol. 25, 822–828 (2010).
Schoeman, J. F., Fieggen, G., Seller, N., Mendelson, M. & Hartzenberg, B. Intractable intracranial tuberculous infection responsive to thalidomide: report of four cases. J. Child Neurol. 21, 301–308 (2006).
Molton, J. S., Huggan, P. J. & Archuleta, S. Infliximab therapy in two cases of severe neurotuberculosis paradoxical reaction. Med. J. Aust. 202, 156–157 (2015).
Lee, J. Y., Yim, J. J. & Yoon, B. W. Adjuvant interferon-γ treatment in two cases of refractory tuberculosis of the brain. Clin. Neurol. Neurosurgery 114, 732–734 (2012).
Misra, U. K., Kalita, J. & Nair, P. P. Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. J. Neurol. Sci. 293, 12–17 (2010).
Schoeman, J. F., Janse van Rensburg, A., Laubscher, J. A. & Springer, P. The role of aspirin in childhood tuberculous meningitis. J. Child Neurol. 26, 956–962 (2011).
Havlir, D. V. et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N. Engl. J. Med. 365, 1482–1491 (2011).
Blanc, F. X. et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N. Engl. J. Med. 365, 1471–1481 (2011).
Torok, M. E. et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV) — associated tuberculous meningitis. Clin. Infect. Dis. 52, 1374–1383 (2011).
Figaji, A. A. & Fieggen, A. G. The neurosurgical and acute care management of tuberculous meningitis: evidence and current practice. Tuberculosis (Edinb.) 90, 393–400 (2010).
Murthy, J. M. Management of intracranial pressure in tuberculous meningitis. Neurocrit. Care 2, 306–312 (2005).
Tai, M. S. & Sharma, V. K. Role of transcranial doppler in the evaluation of vasculopathy in tuberculous meningitis. PLoS ONE 11, e0164266 (2016).
Kilic, T., Elmaci, I., Ozek, M. M. & Pamir, M. N. Utility of transcranial Doppler ultrasonography in the diagnosis and follow-up of tuberculous meningitis-related vasculopathy. Childs Nerv. Syst. 18, 142–146 (2002).
van Toorn, R., Schaaf, H. S., Solomons, R., Laubscher, J. A. & Schoeman, J. F. The value of transcranial Doppler imaging in children with tuberculous meningitis. Childs Nerv. Syst. 30, 1711–1716 (2014).
Figaji, A. A. et al. Continuous monitoring and intervention for cerebral ischemia in tuberculous meningitis. Pediatr. Crit. Care Med. 9, e25–e30 (2008).
Oddo, M. et al. Effect of mannitol and hypertonic saline on cerebral oxygenation in patients with severe traumatic brain injury and refractory intracranial hypertension. J. Neurol. Neurosurg. Psychiatry 80, 916–920 (2009).
Francony, G. et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit. Care Med. 36, 795–800 (2008).
Narotam, P. K. et al. Hyponatremic natriuretic syndrome in tuberculous meningitis: the probable role of atrial natriuretic peptide. Neurosurgery 34, 982–988 (1994).
Sterns, R. H. & Silver, S. M. Cerebral salt wasting versus SIADH: what difference? J. Am. Soc. Nephrol. 19, 194–196 (2008).
Moller, K., Larsen, F. S., Bie, P. & Skinhoj, P. The syndrome of inappropriate secretion of antidiuretic hormone and fluid restriction in meningitis—how strong is the evidence? Scand. J. Infect. Dis. 33, 13–26 (2001).
Schoeman, J., Donald, P., van Zyl, L., Keet, M. & Wait, J. Tuberculous hydrocephalus: comparison of different treatments with regard to ICP, ventricular size and clinical outcome. Dev. Med. Child Neurol. 33, 396–405 (1991).
Visudhiphan, P. & Chiemchanya, S. Hydrocephalus in tuberculous meningitis in children: treatment with acetazolamide and repeated lumbar puncture. J. Pediatr. 95, 657–660 (1979).
Figaji, A. A., Fieggen, A. G. & Peter, J. C. Endoscopic third ventriculostomy in tuberculous meningitis. Childs Nerv. Syst. 19, 217–225 (2003).
Bruwer, G. E., Van der Westhuizen, S., Lombard, C. J. & Schoeman, J. F. Can CT predict the level of CSF block in tuberculous hydrocephalus? Childs Nerv. Syst. 20, 183–187 (2004).
Figaji, A. A., Fieggen, A. G. & Peter, J. C. Re: Endoscopic third ventriculostomy for chronic hydrocephalus after tuberculous meningitis [Jonathan A, Rajshekhar, V Surg Neurol 63 (2005) 32–35]. Surg. Neurol. 64, 95 (2005).
Rizvi, I. et al. Ventriculo-peritoneal shunt surgery for tuberculous meningitis: a systematic review. J. Neurol. Sci. 375, 255–263 (2017).
Sil, K. & Chatterjee, S. Shunting in tuberculous meningitis: a neurosurgeon's nightmare. Childs Nerv. Syst. 24, 1029–1032 (2008).
Figaji, A. A. & Fieggen, A. G. Endoscopic challenges and applications in tuberculous meningitis. World Neurosurg. 79, S24e29–S24e14 (2013).
Goyal, P. et al. A randomized study of ventriculoperitoneal shunt versus endoscopic third ventriculostomy for the management of tubercular meningitis with hydrocephalus. Childs Nerv. Syst. 30, 851–857 (2014).
Lamprecht, D., Schoeman, J., Donald, P. & Hartzenberg, H. Ventriculoperitoneal shunting in childhood tuberculous meningitis. Br. J. Neurosurg. 15, 119–125 (2001).
Sharma, R. M. et al. Tubercular meningitis with hydrocephalus with HIV co-infection: role of cerebrospinal fluid diversion procedures. J. Neurosurg. 122, 1087–1095 (2015).
Yadav, Y. R. et al. Role of endoscopic third ventriculostomy in tuberculous meningitis with hydrocephalus. Asian J. Neurosurgery 11, 325–329 (2016).
Rajshekhar, V. Surgery for brain tuberculosis: a review. Acta Neurochir. (Wien) 157, 1665–1678 (2015).
Kumar, A., Singh, K. & Sharma, V. Surgery in hydrocephalus of tubercular origin: challenges and management. Acta Neurochir. (Wien) 155, 869–873 (2013).
Lawn, S. D. & Wilkinson, R. J. ART and prevention of HIV-associated tuberculosis. Lancet HIV 2, e221–e222 (2015).
Marais, B. J. et al. Standardized methods for enhanced quality and comparability of tuberculous meningitis studies. Clin. Infect. Dis. 64, 501–509 (2017).
Esmail, H. et al. Characterization of progressive HIV-associated tuberculosis using 2-deoxy-2-[18F]fluoro-D-glucose positron emission and computed tomography. Nat. Med. 22, 1090–1093 (2016).
Berry, M. P. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010).
Kumar, G. S. et al. Gene expression profiling of tuberculous meningitis co-infected with HIV. J. Proteom. Bioinform. 5, 235–244 (2012).
Bratton, D. J., Phillips, P. P. & Parmar, M. K. A multi-arm multi-stage clinical trial design for binary outcomes with application to tuberculosis. BMC Med. Res. Methodol. 13, 139 (2013).
Campo, M. et al. Common polymorphisms in the CD43 gene region are associated with tuberculosis disease and mortality. Am. J. Respir. Cell. Mol. Biol. 52, 342–348 (2015).
Hoal-Van Helden, E. G. et al. Mannose-binding protein B allele confers protection against tuberculous meningitis. Pediatr. Res. 45, 459–464 (1999).
Rizvi, I. et al. Vitamin D status, vitamin D receptor and toll like receptor-2 polymorphisms in tuberculous meningitis: a case-control study. Infection 44, 633–640 (2016).
Zhao, Y. et al. Genetic polymorphisms of CCL1 rs2072069 G/A and TLR2 rs3804099 T/C in pulmonary or meningeal tuberculosis patients. Int. J. Clin. Exp. Pathol. 8, 12608–12620 (2015).
Thuong, N. T. et al. A polymorphism in human TLR2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun. 8, 422–428 (2007).
Graustein, A. D. et al. TLR9 gene region polymorphisms and susceptibility to tuberculosis in Vietnam. Tuberculosis (Edinb.) 95, 190–196 (2015).
Dissanayeke, S. R. et al. Polymorphic variation in TIRAP is not associated with susceptibility to childhood TB but may determine susceptibility to TBM in some ethnic groups. PLoS ONE 4, e6698 (2009).
Hawn, T. R. et al. A polymorphism in toll-interleukin 1 receptor domain containing adaptor protein is associated with susceptibility to meningeal tuberculosis. J. Infect. Dis. 194, 1127–1134 (2006).
Horne, D. J. et al. Common polymorphisms in the PKP3–SIGIRR–TMEM16J gene region are associated with susceptibility to tuberculosis. J. Infect. Dis. 205, 586–594 (2012).
Feng, W. X. et al. Tag SNP polymorphism of CCL2 and its role in clinical tuberculosis in Han Chinese pediatric population. PLoS ONE 6, e14652 (2011).
Kumar, R., Singh, S. N. & Kohli, N. A diagnostic rule for tuberculous meningitis. Arch. Dis. Child. 81, 221–224 (1999).
Youssef, F. G. et al. Differentiation of tuberculous meningitis from acute bacterial meningitis using simple clinical and laboratory parameters. Diagn. Microbiol. Infect. Dis. 55, 275–278 (2006).
Cohen, D. B. et al. Diagnosis of cryptococcal and tuberculous meningitis in a resource-limited African setting. Trop. Med. Int. Health 15, 910–917 (2010).
Hristea, A. et al. Clinical prediction rule for differentiating tuberculous from viral meningitis. Int. J. Tuberc. Lung Dis. 16, 793–798 (2012).
Dendane, T. et al. A simple diagnostic aid for tuberculous meningitis in adults in Morocco by use of clinical and laboratory features. Int. J. Infect. Dis. 17, e461–e465 (2013).
Zhang, B., Lv, K., Bao, J., Lu, C. & Lu, Z. Clinical and laboratory factors in the differential diagnosis of tuberculous and cryptococcal meningitis in adult HIV-negative patients. Intern. Med. 52, 1573–1578 (2013).
Qamar, F. N., Rahman, A. J., Iqbal, S. & Humayun, K. Comparison of clinical and CSF profiles in children with tuberculous and pyogenic meningitis; role of CSF protein: glucose ratio as diagnostic marker of tuberculous meningitis. J. Pak. Med. Assoc. 63, 206–210 (2013).
Acknowledgements
Tuberculous Meningitis International Research Consortium members include: Rob Aarnoutse, Reinout Van Crevel, Aarjan van Laarhoven, Sofiati Dian (Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands); Nathan C. Bahr (University of Kansas Medical Center, Kansas City, USA); David R. Boulware (University of Minnesota, Minneapolis, USA); Maxine Caws (Liverpool School of Tropical Medicine, Liverpool, UK); Mark R. Cronan, David Tobin (Duke University School of Medicine, Durham, USA); Kelly Dooley (Johns Hopkins University School of Medicine, Baltimore, USA); Sarah Dunstan (University of Melbourne, Melbourne, Australia); Guo-dong Feng, Xiaodan Shi, Ting Wang (Fourth Military Medical University, Xi'an, People's Republic of China); Anthony Figaji, Suzaan Marais, Helen McIlleron, Graeme Meintjes, Ursula Rohlwink (University of Cape Town, Cape Town, South Africa); Ahmad Rizal, Rovina Ruslami (Padjadjaran University, Bandung, Indonesia); Ravindra K. Garg (King George Medical University, Lucknow, India); Mudit Gupta, Rakesh K. Gupta (Fortis Memorial Research Institute, Gurgaon, India); Sneha Gupta, Rada Savic (University of California, San Francisco, USA); Anna D. Heemskerk, Thuong Thuy Thuong Nguyễn, Mai Thi Hoàng Nguyễn, Vijay Srinivasan, Guy Thwaites, Trâm Thi Bích Trân, Thinh Thi Vân Trân, Anh Thi Ngoc Trân, Trang Hồng Yêng Võ, Marcel Wolbers (Oxford University Clinical Research Unit, Ho Chi Minh City, Vietnam); Jayantee Kalita, Usha K. Misra (Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India); Rachel Lai (The Francis Crick Institute, London, UK); Ben J. Marais, Mai Quỳnh Trinh (University of Sydney, Sydney, Australia); Bằng Đức Nguyễn, Yến Bích Nguyễn (Pham Ngoc Thach Hospital for Tuberculosis & Lung Diseases, Ho Chi Minh City, Vietnam); Vinod Patel (University of KwaZulu-Natal, Durban, South Africa); Thomas Pouplin (Mahidol-Oxford Tropical Medicine Research Unit, Bangkok, Thailand); Lalita Ramakrishnan (University of Cambridge, Cambridge, UK); Johan F. Schoeman, Regan Solomons, Ronald Van Toorn (University of Stellenbosch, Cape Town, South Africa); James Seddon (Imperial College, London, UK); Javeed Shah (University of Washington, Washington, USA); Jaya S. Tyagi (All India Institute of Medical Sciences, New Delhi, India); Douwe H. Visser (VU University Medical Center, Amsterdam, The Netherlands); Robert J. Wilkinson (Imperial College and The Francis Crick Institute, London, UK and University of Cape Town, South Africa). R.J.W. is supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC00110218), the UK Medical Research Council (FC00110218), and the Wellcome Trust (FC00110218). He also receives support from the Wellcome Trust (104803, 203135) and the National Research Foundation Of South Africa (96841). G.T. is supported by the Wellcome Trust through a Major Overseas Programme grant (106680/Z/14/Z) and an Investigator Award (110179/Z/15/Z).
Author information
Authors and Affiliations
Consortia
Contributions
R.J.W, U.R., U.K.M., R.v.C., N.T.H.M., K.E.D., M.C., A.F., and G.T. researched data for the article, R.J.W, U.R., U.K.M., R.v.C., K.E.D., M.C., A.F., R. Savic, R. Solomons, and G.T made a substantial contribution to discussion of content, R.J.W., U.R., U.K.M., R.v.C., wrote the article, and all authors contributed to the review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Extrapulmonary
-
Tuberculosis occurring outside the lungs.
- Miliary
-
Disseminated micronodular tuberculosis of the lungs.
- Rich focus
-
The initial intracranial lesion of tuberculous meningitis, as described by Arnold Rich.
- Tuberculoma
-
A clinical manifestation of tuberculosis in which tubercles comglomerate into a firm lump, and so can mimic cancer tumours of many types in medical imaging studies.
- Basal exudates
-
An inflammatory reaction to tuberculosis in the basal cisterns of the brain.
- Paradoxical worsening
-
The worsening of a tuberculosis lesion during otherwise effective antirtubercular or antiretroviral therapy.
- Ziehl–Neelsen staining
-
A technique to visualize Mycobacterium tuberculosis directly by microscopy in pathological samples.
- Paucibacillary
-
Disease associated with very low numbers of bacteria in clinical specimens.
Rights and permissions
About this article
Cite this article
Wilkinson, R., Rohlwink, U., Misra, U. et al. Tuberculous meningitis. Nat Rev Neurol 13, 581–598 (2017). https://doi.org/10.1038/nrneurol.2017.120
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2017.120
This article is cited by
-
A retrospective analysis of 20,178 adult neurological infection admissions to United Kingdom critical care units from 2001 to 2020
BMC Infectious Diseases (2024)
-
Case-controlled study of tuberculosis in in-vitro fertilisation-embryo transfer and natural pregnancy
BMC Pregnancy and Childbirth (2024)
-
Therapeutic developments for tuberculosis and nontuberculous mycobacterial lung disease
Nature Reviews Drug Discovery (2024)
-
Ventriculoperitoneal shunt for tuberculous meningitis-associated hydrocephalus: long-term outcomes and complications
BMC Infectious Diseases (2023)
-
CNS Infections in Patients Living with HIV/AIDS
Current Tropical Medicine Reports (2023)