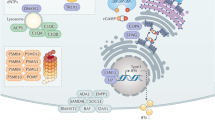
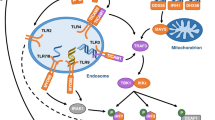
Key Points
-
Type I interferon is an essential component of the innate immune system, which protects the brain against viral infection
-
Underactivity of the type I interferon pathway causes predisposition to herpes simplex encephalitis
-
Overactivity of the type I interferon pathway results in a spectrum of Mendelian neuroinflammatory diseases known as 'interferonopathies'
-
Dysregulation of the type I interferon response has an important role in the pathogenesis of more common diseases that can affect the brain, including lupus and cerebrovascular disease
-
Aberrant activation of the type I interferon pathway offers potential as a useful biomarker and a target for therapy
Abstract
Type I interferon is an essential component of the brain's innate immune defence, conferring protection against viral infection. Recently, dysregulation of the type I interferon pathway has been implicated in the pathogenesis of a spectrum of neuroinfectious and neuroinflammatory disorders. Underactivity of the type I interferon response is associated with a predisposition to herpes simplex encephalitis. Conversely, a group of 'interferonopathic' disorders, characterized by severe neuroinflammation and overactivity of type I interferon, has been described. Elucidation of the genetic basis of these Mendelian neuroinflammatory diseases has uncovered important links between nucleic acid sensors, innate immune activation and neuroinflammatory disease. These mechanisms have an important role in the pathogenesis of more common polygenic diseases that can affect the brain, such as lupus and cerebral small vessel disease. In this article, we review the spectrum of neurological disease associated with type I interferon dysregulation, as well as advances in our understanding of the molecular and cellular pathogenesis of these conditions. We highlight the potential utility of type I interferon as both a biomarker and a therapeutic target in neuroinflammatory disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Isaacs, A. & Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147, 258–267 (1957).
Isaacs, A., Lindenmann, J. & Valentine, R. C. Virus interference. II. Some properties of interferon. Proc. R. Soc. Lond. B Biol. Sci. 147, 268–273 (1957).
Nagano, Y., Kojima, Y. & Sawai, Y. Immunity and interference in vaccinia; inhibition of skin infection by inactivated virus [French]. C. R. Seances Soc. Biol. Fil. 148, 750–752 (1954).
Wheelock, E. F. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science 149, 310–311 (1965).
Field, A. K., Tytell, A. A., Lampson, G. P. & Hilleman, M. R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc. Natl Acad. Sci. USA 58, 1004–1010 (1967).
Crow, Y. J. Type I interferonopathies: a novel set of inborn errors of immunity. Ann. N. Y. Acad. Sci. 1238, 91–98 (2011).
Olopade, O. I. et al. Mapping of the shortest region of overlap of deletions of the short arm of chromosome 9 associated with human neoplasia. Genomics 14, 437–443 (1992).
Altmann, S. M., Mellon, M. T., Distel, D. L. & Kim, C. H. Molecular and functional analysis of an interferon gene from the zebrafish, Danio rerio. J. Virol. 77, 1992–2002 (2003).
Robertsen, B., Bergan, V., Rokenes, T., Larsen, R. & Albuquerque, A. Atlantic salmon interferon genes: cloning, sequence analysis, expression, and biological activity. J. Interferon Cytokine Res. 23, 601–612 (2003).
Xu, L., Yang, L. & Liu, W. Distinct evolution process among type I interferon in mammals. Protein Cell 4, 383–392 (2013).
Kerkmann, M. et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J. Immunol. 170, 4465–4474 (2003).
Takauji, R. et al. CpG-DNA-induced IFN-α production involves p38 MAPK-dependent STAT1 phosphorylation in human plasmacytoid dendritic cell precursors. J. Leukoc. Biol. 72, 1011–1019 (2002).
Izaguirre, A. et al. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74, 1125–1138 (2003).
Desmet, C. J. & Ishii, K. J. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat. Rev. Immunol. 12, 479–491 (2012).
Oldenburg, M. et al. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science 337, 1111–1115 (2012).
Rigby, R. E. et al. RNA:DNA hybrids are a novel molecular pattern sensed by TLR9. EMBO J. 33, 542–558 (2014).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 45 5, 674–678 (2008).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
de Weerd, N. A., Samarajiwa, S. A. & Hertzog, P. J. Type I interferon receptors: biochemistry and biological functions. J. Biol. Chem. 282, 20053–20057 (2007).
van Boxel-Dezaire, A. H., Rani, M. R. & Stark, G. R. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25, 361–372 (2006).
Schoggins, J. W. & Rice, C. M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 1, 519–525 (2011).
Schoggins, J. W. et al. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472, 481–485 (2011).
Ma, F. et al. Positive feedback regulation of type I interferon by the interferon-stimulated gene STING. EMBO Rep. 16, 202–212 (2015).
Kim, M.-J., Hwang, S.-Y., Imaizumi, T. & Yoo, J.-Y. Negative feedback regulation of RIG-I-mediated antiviral signaling by interferon-induced ISG15 conjugation. J. Virol. 82, 1474–1483 (2008).
Staeheli, P., Pitossi, F. & Pavlovic, J. Mx proteins: GTPases with antiviral activity. Trends Cell Biol. 3, 268–272 (1993).
Zurcher, T., Pavlovic, J. & Staeheli, P. Mechanism of human MxA protein action: variants with changed antiviral properties. EMBO J. 11, 1657–1661 (1992).
Haller, O. & Kochs, G. Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity. J. Interferon Cytokine Res. 31, 79–87 (2011).
Kochs, G., Janzen, C., Hohenberg, H. & Haller, O. Antivirally active MxA protein sequesters La Crosse virus nucleocapsid protein into perinuclear complexes. Proc. Natl Acad. Sci. USA 99, 3153–3158 (2002).
Kochs, G., Haener, M., Aebi, U. & Haller, O. Self-assembly of human MxA GTPase into highly ordered dynamin-like oligomers. J. Biol. Chem. 277, 14172–14176 (2002).
Reichelt, M., Stertz, S., Krijnse-Locker, J., Haller, O. & Kochs, G. Missorting of LaCrosse virus nucleocapsid protein by the interferon-induced MxA GTPase involves smooth ER membranes. Traffic 5, 772–784 (2004).
Samarajiwa, S. A., Forster, S., Auchettl, K. & Hertzog, P. J. INTERFEROME: the database of interferon regulated genes. Nucleic Acids Res. 37, D852–D857 (2009).
Lafaille, F. G. et al. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491, 769–773 (2012).
Muller, U. et al. Functional role of type I and type II interferons in antiviral defense. Science 264, 1918–1921 (1994).
Detje, C. N. et al. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J. Immunol. 18 2, 2297–2304 (2009).
Detje, C. N. et al. Upon intranasal vesicular stomatitis virus infection, astrocytes in the olfactory bulb are important interferon Beta producers that protect from lethal encephalitis. J. Virol. 89, 2731–2738 (2015).
Campbell, I. L. et al. Structural and functional neuropathology in transgenic mice with CNS expression of IFN-α. Brain Res. 835, 46–61 (1999).
Akwa, Y. et al. Transgenic expression of IFN-α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J. Immunol. 161, 5016–5026 (1998).
Granerod, J. et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect. Dis. 10, 835–844 (2010).
Smith, M. G., Lennette, E. H. & Reames, H. R. Isolation of the virus of herpes simplex and the demonstration of intranuclear inclusions in a case of acute encephalitis. Am. J. Pathol. 17, 55–681 (1941).
Whitley, R. J. Herpes simplex virus in children. Curr. Treat. Options Neurol. 4, 231–237 (2002).
Najioullah, F. et al. Diagnosis and surveillance of herpes simplex virus infection of the central nervous system. J. Med. Virol. 61, 468–473 (2000).
Nahmias, A. J., Lee, F. K. & Beckman-Nahmias, S. Sero-epidemiological and -sociological patterns of herpes simplex virus infection in the world. Scand. J. Infect. Dis. Suppl. 69, 19–36 (1990).
Lerner, A. M., Levine, D. P. & Reyes, M. P. Two cases of herpes simplex virus encephalitis in the same family. N. Engl. J. Med. 308, 1481 (1983).
Jackson, A. C., Melanson, M. & Rossiter, J. P. Familial herpes simplex encephalitis. Ann. Neurol. 51, 406–407 (2002).
Koskiniemi, M. et al. Familial herpes encephalitis. Lancet 346, 1553 (1995).
Abel, L. et al. Age-dependent Mendelian predisposition to herpes simplex virus type 1 encephalitis in childhood. J. Pediatr. 157, 623–629, 629.e1 (2010).
Dupuis, S. et al. Impaired response to interferon-α/β and lethal viral disease in human STAT1 deficiency. Nat. Genet. 33, 388–391 (2003).
Kong, X. F. et al. A novel form of human STAT1 deficiency impairing early but not late responses to interferons. Blood 116, 5895–5906 (2010).
Casrouge, A. et al. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314, 308–312 (2006).
Kim, Y. M., Brinkmann, M. M., Paquet, M. E. & Ploegh, H. L. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452, 234–238 (2008).
Zhang, S. Y. et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science 31 7, 1522–1527 (2007).
Guo, Y. et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J. Exp. Med. 208, 2083–2098 (2011).
Aksentijevich, I. et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N. Engl. J. Med. 360, 2426–2437 (2009).
Aicardi, J. & Goutières, F. A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann. Neurol. 15, 49–54 (1984).
Lebon, P. et al. Intrathecal synthesis of interferon-alpha in infants with progressive familial encephalopathy. J. Neurol. Sci. 84, 201–208 (1988).
Rice, G. I. et al. Assessment of interferon-related biomarkers in Aicardi–Goutières syndrome associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, and ADAR: a case–control study. Lancet Neurol. 12, 1159–1169 (2013).
Crow, Y. J. et al. Mutations in the gene encoding the 3′–5′ DNA exonuclease TREX1 cause Aicardi–Goutières syndrome at the AGS1 locus. Nat. Genet. 38, 917–920 (2006).
Crow, Y. J. et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi–Goutières syndrome and mimic congenital viral brain infection. Nat. Genet. 38, 910–916 (2006).
Rice, G. I. et al. Mutations involved in Aicardi–Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41, 829–832 (2009).
Goldstone, D. C. et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480, 379–382 (2011).
Alarcon-Riquelme, M. E. Nucleic acid by-products and chronic inflammation. Nat. Genet. 38, 866–867 (2006).
Rigby, R. E., Leitch, A. & Jackson, A. P. Nucleic acid-mediated inflammatory diseases. Bioessays 30, 833–8 (2008).
Yang, Y. G., Lindahl, T. & Barnes, D. E. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 13 1, 873–886 (2007).
Rice, G. I. et al. Mutations in ADAR1 cause Aicardi–Goutières syndrome associated with a type I interferon signature. Nat. Genet. 44, 1243–1248 (2012).
Livingston, J. H. et al. A type I interferon signature identifies bilateral striatal necrosis due to mutations in ADAR1. J. Med. Genet. 51, 76–82 (2014).
Mannion, N. M. et al. The RNA-editing enzyme ADAR1 controls innate immune responses to RNA. Cell Rep. 9, 1482–1494 (2014).
Rice, G. I. et al. Gain-of-function mutations in IFIH1 cause a spectrum of human disease phenotypes associated with upregulated type I interferon signaling. Nat. Genet. 46, 503–509 (2014).
Rice, G. et al. Clinical and molecular phenotype of Aicardi–Goutières syndrome. Am. J. Hum. Genet. 81, 713–725 (2007).
Ramesh, V. et al. Intracerebral large artery disease in Aicardi–Goutières syndrome implicates SAMHD1 in vascular homeostasis. Dev. Med. Child Neurol. 5 2, 725–732 (2010).
Crow, Y. J. et al. Cree encephalitis is allelic with Aicardi–Goutières syndrome: implications for the pathogenesis of disorders of interferon alpha metabolism. J. Med. Genet. 4 0, 183–187 (2003).
Zhang, X. et al. Human intracellular ISG15 prevents interferon-α/β over-amplification and auto-inflammation. Nature 517, 89–93 (2015).
Livingston, J. H., Stivaros, S., van der Knaap, M. S. & Crow, Y. J. Recognizable phenotypes associated with intracranial calcification. Dev. Med. Child Neurol. 55, 46–57 (2013).
Goldmann, T. et al. USP18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J. 34, 1612–1629 (2015).
Rice, G. et al. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi–Goutières syndrome. Am. J. Hum. Genet. 80, 811–815 (2007).
Navarro, V. et al. Two further cases of spondyloenchondrodysplasia (SPENCD) with immune dysregulation. Am. J. Med. Genet. A 146A, 2810–2815 (2008).
Briggs, T. A. et al. Tartrate-resistant acid phosphatase deficiency causes a bone dysplasia with autoimmunity and a type I interferon expression signature. Nat. Genet. 43, 127–131 (2011).
Liu, Y. et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 37 1, 507–518 (2014).
Lisnevskaia, L., Murphy, G. & Isenberg, D. Systemic lupus erythematosus. Lancet 384, 1878–1888 (2014).
Botto, M. et al. Complement in human diseases: Lessons from complement deficiencies. Mol. Immunol. 46, 2774–2783 (2009).
Banchereau, J. & Pascual, V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity 25, 383–392 (2006).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN) et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat. Genet. 40, 204–210 (2008).
Gateva, V. et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus. Nat. Genet. 41, 1228–1233 (2009).
Dale, R. C., Tang, S. P., Heckmatt, J. Z. & Tatnall, F. M. Familial systemic lupus erythematosus and congenital infection-like syndrome. Neuropediatrics 31, 155–158 (2000).
De Laet, C. et al. Phenotypic overlap between infantile systemic lupus erythematosus and Aicardi–Goutières syndrome. Neuropediatrics 36, 399–402 (2005).
Lee-Kirsch, M. A. et al. A mutation in TREX1 that impairs susceptibility to granzyme A-mediated cell death underlies familial chilblain lupus. J. Mol. Med. (Berl.) 85, 531–537 (2007).
Lee-Kirsch, M. A. et al. Mutations in the gene encoding the 3′–5′ DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat. Genet. 39, 1065–1067 (2007).
Namjou, B. et al. Evaluation of the TREX1 gene in a large multi-ancestral lupus cohort. Genes Immun. 12, 270–279 (2011).
de Vries, B. et al. TREX1 gene variant in neuropsychiatric systemic lupus erythematosus. Ann. Rheum. Dis. 69, 1886–1887 (2010).
Günther, C. et al. Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J. Clin. Invest. 125, 413–424 (2015).
Reijns, M. A. et al. Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell 149, 1008–1022 (2012).
Tan, F. K. et al. Signatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patients. Rheumatology (Oxford) 45, 694–702 (2006).
Barth, P. G., Walter, A. & van Gelderen, I. Aicardi–Goutières syndrome: a genetic microangiopathy? Acta Neuropathol. 98, 212–216 (1999).
Rasmussen, M., Skullerud, K., Bakke, S. J., Lebon, P. & Jahnsen, F. L. Cerebral thrombotic microangiopathy and antiphospholipid antibodies in Aicardi–Goutières syndrome—report of two sisters. Neuropediatrics 36, 40–44 (2005).
Hanly, J. G., Walsh, N. M. & Sangalang, V. Brain pathology in systemic lupus erythematosus. J. Rheumatol. 19, 732–741 (1992).
Richards, A. et al. C-terminal truncations in human 3′–5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat. Genet. 3 9, 1068–1070 (2007).
Kolar, G. R. et al. Neuropathology and genetics of cerebroretinal vasculopathies. Brain Pathol. 24, 510–518 (2014).
Mateen, F. J. et al. Evolution of a tumor-like lesion in cerebroretinal vasculopathy and TREX1 mutation. Neurology 75, 1211–1213 (2010).
Schuh, E. et al. Multiple sclerosis-like lesions and type I interferon signature in a patient with RVCL. Neurol. Neuroimmunol. Neuroinflamm. 2, e55 (2015).
Pelzer, N. et al. Heterozygous TREX1 mutations in early-onset cerebrovascular disease. J. Neurol. 260, 2188–2190 (2013).
Magee, C. C. Renal thrombotic microangiopathy induced by interferon-alpha. Nephrol. Dial. Transplant. 16, 2111–2112 (2001).
Larochelle, C. et al. Thrombotic thrombocytopenic purpura–hemolytic uremic syndrome in relapsing-remitting multiple sclerosis patients on high-dose interferon β. Mult. Scler. 2 0, 1783–1787 (2014).
Hunt, D. et al. Thrombotic microangiopathy associated with interferon beta. N. Engl. J. Med. 370, 1270–1271 (2014).
Magro, C. M. et al. Degos disease: a C5b-9/interferon-α-mediated endotheliopathy syndrome. Am. J. Clin. Pathol. 135, 599–610 (2011).
Crow, Y. J. & Manel, N. Aicardi–Goutières syndrome and the type I interferonopathies. Nat. Rev. Immunol. 15, 429–440 (2015).
Cuadrado, E. et al. Phenotypic variation in Aicardi–Goutières syndrome explained by cell-specific IFN-stimulated gene response and cytokine release. J. Immunol. 194, 3623–3633 (2015).
van Heteren, J. T. et al. Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in Aicardi–Goutières syndrome. Glia 56, 568–578 (2008).
Stetson, D. B., Ko, J. S., Heidmann, T. & Medzhitov, R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598 (2008).
Gall, A. et al. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity 36, 120–131 (2012).
Morita, M. et al. Gene-targeted mice lacking the Trex1 (DNase III) 3′→5′ DNA exonuclease develop inflammatory myocarditis. Mol. Cell. Biol. 24, 6719–6727 (2004).
Beck-Engeser, G. B., Eilat, D. & Wabl, M. An autoimmune disease prevented by anti-retroviral drugs. Retrovirology 8, 91 (2011).
Volkman, H. E. & Stetson, D. B. The enemy within: endogenous retroelements and autoimmune disease. Nat. Immunol. 15, 415–422 (2014).
Petri, M. et al. Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: a phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 65, 1011–1021 (2013).
Merrill, J. T. et al. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon α monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Ann. Rheum. Dis. 70, 1905–1913 (2011).
Crow, Y. J. et al. Characterization of human disease phenotypes associated with mutations in TREX1, RNASEH2A, RNASEH2B, RNASEH2C, SAMHD1, ADAR, and IFIH1. Am. J. Med. Genet. A 167A, 296–312 (2015).
Polman, C. H. et al. Recommendations for clinical use of data on neutralising antibodies to interferon-beta therapy in multiple sclerosis. Lancet Neurol. 9, 740–750 (2010).
Klippel, J. H., Carette, S., Preble, O. T., Friedman, R. M. & Grimley, P. M. Serum alpha interferon and lymphocyte inclusions in systemic lupus erythematosus. Ann. Rheum. Dis. 44, 104–108 (1985).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content, wrote the article, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
McGlasson, S., Jury, A., Jackson, A. et al. Type I interferon dysregulation and neurological disease. Nat Rev Neurol 11, 515–523 (2015). https://doi.org/10.1038/nrneurol.2015.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2015.143
This article is cited by
-
Granulocyte-macrophage colony-stimulating factor suppresses induction of type I interferon in infants with severe pneumonia
Pediatric Research (2023)
-
Glioprotective effects of resveratrol in hypothalamic astrocyte cultures obtained from interferon receptor knockout (IFNα/βR−/−) mice
In Vitro Cellular & Developmental Biology - Animal (2023)
-
Emerging concepts of type I interferons in SLE pathogenesis and therapy
Nature Reviews Rheumatology (2022)
-
A novel phosphoproteomic landscape evoked in response to type I interferon in the brain and in glial cells
Journal of Neuroinflammation (2021)
-
Mice defective in interferon signaling help distinguish between primary and secondary pathological pathways in a mouse model of neuronal forms of Gaucher disease
Journal of Neuroinflammation (2020)