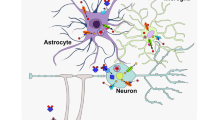
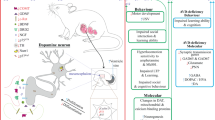
Abstract
The effects of iron deficiency are well documented, but relatively little is known about the long-term implications of iron overload during development. High levels of redox-active iron in the brain have been associated with neurodegenerative disorders, most notably Parkinson disease, yet a gradual increase in brain iron seems to be a feature of normal ageing. Increased brain iron levels might result from intake of infant formula that is excessively fortified with iron, thereby altering the trajectory of brain iron uptake and amplifying the risk of iron-associated neurodegeneration in later life. In this Perspectives article, we discuss the potential long-term implications of excessive iron intake in early life, propose the analysis of iron deposits in teeth as a method for retrospective determination of iron exposure during critical developmental windows, and call for evidence-based optimization of the chemical composition of infant dietary supplements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Beard, J. L. Iron biology in immune function, muscle metabolism and neuronal functioning. J. Nutr. 131, 568S–579S (2001).
Weinberg, E. D. The Lactobacillus anomaly: total iron abstinence. Perspect. Biol. Med. 40, 578–583 (1997).
Aguirre, J. D. et al. A manganese-rich environment supports superoxide dismutase activity in a Lyme disease pathogen, Borrelia burgdorferi. J. Biol. Chem. 288, 8468–8478 (2013).
Beard, J. L., Connor, J. R. & Jones, B. C. Iron in the brain. Nutr. Rev. 51, 157–170 (1993).
Beard, J. Iron deficiency alters brain development and functioning. J. Nutr. 133, 1468S–1472S (2003).
Ziegler, E. E., Nelson, S. E. & Jeter, J. M. Iron supplementation of breastfed infants from an early age. Am. J. Clin. Nutr. 89, 525–532 (2009).
Lozoff, B. & Georgieff, M. K. Iron deficiency and brain development. Semin. Pediatr. Neurol. 13, 158–165 (2006).
Carter, R. C. et al. Iron deficiency anemia and cognitive function in infancy. Pediatrics 126, e427–e434 (2010).
World Health Organization. Worldwide prevalence of anaemia 1993–2005: WHO global database on anaemia. World Health Organization Institutional Repository for Information Sharing [online], (2008).
Zimmermann, M. B. & Hurrell, R. F. Nutritional iron deficiency. Lancet 370, 511–520 (2007).
Horta, B., Victora, C. & World Health Organization. Long-term effects of breastfeeding: a systematic review. World Health Organization Institutional Repository for Information Sharing [online]. (2013).
Ibanez, G. et al. Prevalence of breastfeeding in industrialized countries. Rev. Epidemiol. Sante Publique 60, 305–320 (2012).
Kong, S. K. & Lee, D. T. Factors influencing decision to breastfeed. J. Adv. Nurs. 46, 369–379 (2004).
Obladen, M. Historic records on the commercial production of infant formula. Neonatology 106, 173–180 (2014).
Forsyth, S. Non-compliance with the International Code of Marketing of Breast Milk Substitutes is not confined to the infant formula industry. J. Public Health (Oxf.) 35, 185–190 (2013).
Lucas, A. et al. Efficacy and safety of long-chain polyunsaturated fatty acid supplementation of infant-formula milk: a randomised trial. Lancet 354, 1948–1954 (1999).
Saarinen, U. M. Need for iron supplementation in infants on prolonged breast feeding. J. Pediatr. 93, 177–180 (1978).
Siimes, M. A., Salmenperä, L. & Perheentupa, J. Exclusive breast-feeding for 9 months: risk of iron deficiency. J. Pediatr. 104, 196–199 (1984).
Lowe, C. U. et al. Iron balance and requirements in infancy. Pediatrics 43, 134–142 (1969).
McMillan, J. A., Landaw, S. A. & Oski, F. A. Iron sufficiency in breast-fed infants and the availability of iron from human milk. Pediatrics 58, 686–691 (1976).
[No authors listed] American Academy of Pediatrics Committee on Nutrition: Iron-fortified infant formulas. Pediatrics 84, 1114–1115 (1989).
[No authors listed] Iron fortification of infant formulas. American Academy of Pediatrics. Committee on Nutrition. Pediatrics 104, 119–123 (1999).
Baker, R. D., Greer, F. R. & Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics 126, 1040–1050 (2010).
Koletzko, B. et al. Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 41, 584–599 (2005).
Domellöf, M. et al. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 58, 119–129 (2014).
MacLean, W. C. Jr et al. Upper levels of nutrients in infant formulas: comparison of analytical data with the revised Codex infant formula standard. J. Food Comp. Anal. 23, 44–53 (2010).
Singhal, A. et al. Clinical safety of iron-fortified formulas. Pediatrics 105, E38 (2000).
Sachdev, H., Gera, T. & Nestel, P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutr. 8, 117–132 (2005).
Friel, J. K. et al. A double-masked, randomized control trial of iron supplementation in early infancy in healthy term breast-fed infants. J. Pediatr. 143, 582–586 (2003).
Berglund, S., Westrup, B. & Domellöf, M. Iron supplements reduce the risk of iron deficiency anemia in marginally low birth weight infants. Pediatrics 126, e874–e883 (2010).
Lozoff, B., Castillo, M., Clark, K. M. & Smith, J. B. Iron-fortified vs low-iron infant formula: developmental outcome at 10 years. Arch. Pediatr. Adolesc. Med. 166, 208–215 (2012).
Hernell, O. & Lönnerdal, B. Recommendations on iron questioned. Pediatrics 127, e1099–e1101 (2011).
Furman, L. M. Exclusively breastfed infants: iron recommendations are premature. Pediatrics 127, e1098–e1099 (2011).
AAP Section on Breastfeeding et al. Concerns with early universal iron supplementation of breastfeeding infants. Pediatrics 127, e1097 (2011).
de la Flor St Remy, R. R., Sánchez, M. L., Sastre, J. B. & Sanz-Medel, A. Multielemental distribution patterns in premature human milk whey and pre-term formula milk whey by size exclusion chromatography coupled to inductively coupled plasma mass spectrometry with octopole reaction cell. J. Anal. At. Spectrom. 19, 1104–1110 (2004).
Arosio, P., Ferrero, R. & Ponzone, A. Ferritin in human milk. Acta Paediatr. Scand. 73, 271–272 (1984).
Lönnerdal, B. Bioactive proteins in breast milk. J. Paediatr. Child Health 49 (Suppl. 1), 1–7 (2013).
Lönnerdal, B. Infant formula and infant nutrition: bioactive proteins of human milk and implications for composition of infant formulas. Am. J. Clin. Nutr. 99, 712S–717S (2014).
Domellöf, M., Lönnerdal, B., Dewey, K. G., Cohen, R. J. & Hernell, O. Iron, zinc, and copper concentrations in breast milk are independent of maternal mineral status. Am. J. Clin. Nutr. 79, 111–115 (2004).
Rai, D. et al. Longitudinal changes in lactoferrin concentrations in human milk: a global systematic review. Crit. Rev. Food Sci. Nutr. 54, 1539–1547 (2014).
Davidsson, L., Kastenmayer, P., Yuen, M., Lönnerdal, B. O. & Hurrell, R. F. Influence of lactoferrin on iron absorption from human milk in infants. Pediatr. Res. 35, 117–124 (1994).
Fairweather-Tait, S. J., Balmer, S. E., Scott, P. H. & Minski, M. J. Lactoferrin and iron absorption in newborn infants. Pediatr. Res. 22, 651–654 (1987).
Stekel, A. et al. Absorption of fortification iron from milk formulas in infants. Am. J. Clin. Nutr. 43, 917–922 (1986).
Abrams, S. A., Wen, J. & Stuff, J. E. Absorption of calcium, zinc, and iron from breast milk by five- to seven-month-old infants. Pediatr. Res. 41, 384–390 (1997).
Mainous, A. G. 3rd, Wells, B., Carek, P. J., Gill, J. M. & Geesey, M. E. The mortality risk of elevated serum transferrin saturation and consumption of dietary iron. Ann. Fam. Med. 2, 139–144 (2004).
Acikyol, B. et al. Brain transcriptome perturbations in the transferrin receptor 2 mutant mouse support the case for brain changes in iron loading disorders, including effects relating to long-term depression and long-term potentiation. Neuroscience 235, 119–128 (2013).
Nandar, W. & Connor, J. R. HFE gene variants affect iron in the brain. J. Nutr. 141, 29S–739S (2011).
Sobotka, T. J. et al. Neurobehavioral dysfunctions associated with dietary iron overload. Physiol. Behav. 59, 213–219 (1996).
Fredriksson, A., Schröder, N., Eriksson, P., Izquierdo, I. & Archer, T. Neonatal iron exposure induces neurobehavioural dysfunctions in adult mice. Toxicol. Appl. Pharmacol. 159, 25–30 (1999).
Piñero, D. J., Li, N. Q., Connor, J. R. & Beard, J. L. Variations in dietary iron alter brain iron metabolism in developing rats. J. Nutr. 130, 254–263 (2000).
Dornelles, A. S. et al. mRNA expression of proteins involved in iron homeostasis in brain regions is altered by age and by iron overloading in the neonatal period. Neurochem. Res. 35, 564–571 (2010).
Miwa, C. P. et al. Neonatal iron treatment increases apoptotic markers in hippocampal and cortical areas of adult rats. Neurotox. Res. 19, 527–535 (2011).
Fernandez, L. L. et al. Early post-natal iron administration induces astroglial response in the brain of adult and aged rats. Neurotox. Res. 20, 193–199 (2011).
Saarinen, U. M., Siimes, M. A. & Dallman, P. R. Iron absorption in infants: high bioavailability of breast milk iron as indicated by the extrinsic tag method of iron absorption and by the concentration of serum ferritin. J. Pediatr. 91, 36–39 (1977).
Lönnerdal, B. & Bryant, A. Absorption of iron from recombinant human lactoferrin in young US women. Am. J. Clin. Nutr. 83, 305–309 (2006).
Rao, R. et al. Iron supplementation dose for perinatal iron deficiency differentially alters the neurochemistry of the frontal cortex and hippocampus in adult rats. Pediatr. Res. 73, 31–37 (2013).
Unger, E. L. et al. Behavior and monoamine deficits in prenatal and perinatal iron deficiency are not corrected by early postnatal moderate-iron or high-iron diets in rats. J. Nutr. 142, 2040–2049 (2012).
Lugonja, N. et al. Differences in direct pharmacologic effects and antioxidative properties of mature breast milk and infant formulas. Nutrition 29, 431–435 (2013).
Zecca, L., Youdim, M. B., Riederer, P., Connor, J. R. & Crichton, R. R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 5, 863–873 (2004).
Ghadery, C. et al. R2* mapping for brain iron: associations with cognition in normal aging. Neurobiol. Aging 36, 925–932 (2015).
Rodrigue, K. M., Haacke, E. M. & Raz, N. Differential effects of age and history of hypertension on regional brain volumes and iron. Neuroimage 54, 750–759 (2011).
Callaghan, M. F. et al. Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiol. Aging 35, 1862–1872 (2014).
Ward, R. J., Zucca, F. A., Duyn, J. H., Crichton, R. R. & Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 13, 1045–1060 (2014).
Berg, D. & Youdim, M. B. Role of iron in neurodegenerative disorders. Top. Magn. Reson. Imaging 17, 5–17 (2006).
Berg, D. et al. Brain iron pathways and their relevance to Parkinson's disease. J. Neurochem. 79, 225–236 (2001).
Lhermitte, J., Kraus, W. M. & McAlpine, D. On the occurrence of abnormal deposits of iron in the brain in parkinsonism with special reference to its localisation. J. Neurol. Psychopathol. 5, 195–208 (1924).
Sulzer, D. et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl Acad. Sci. USA 97, 11869–11874 (2000).
Zecca, L. et al. Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour. Mol. Pathol. 54, 414–418 (2001).
Bohic, S. et al. Intracellular chemical imaging of the developmental phases of human neuromelanin using synchrotron X-ray microspectroscopy. Anal. Chem. 80, 9557–9566 (2008).
Lei, P. et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat. Med. 18, 291–295 (2012).
Ayton, S. et al. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann. Neurol. 73, 554–559 (2013).
Jin, L. et al. Decreased serum ceruloplasmin levels characteristically aggravate nigral iron deposition in Parkinson's disease. Brain 134, 50–58 (2011).
Wang, Z. et al. DJ-1 modulates the expression of Cu/Zn-superoxide dismutase-1 through the Erk1/2-Elk1 pathway in neuroprotection. Ann. Neurol. 70, 591–599 (2011).
Rouault, T. A. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat. Rev. Neurosci. 14, 551–564 (2013).
Lovell, M. A., Robertson, J. D., Teesdale, W. J., Campbell, J. L. & Markesbery, W. R. Copper, iron and zinc in Alzheimer's disease senile plaques. J. Neurol. Sci. 158, 47–52 (1998).
Smith, M. A., Harris, P. L., Sayre, L. M. & Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl Acad. Sci. USA 94, 9866–9868 (1997).
Schrag, M., Mueller, C., Oyoyo, U., Smith, M. A. & Kirsch, W. M. Iron, zinc and copper in the Alzheimer's disease brain: a quantitative meta-analysis. Some insight on the influence of citation bias on scientific opinion. Prog. Neurobiol. 94, 296–306 (2011).
Duce, J. A. et al. Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell 142, 857–867 (2010).
Smith, M. A. et al. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J. Alzheimers Dis. 19, 363–372 (2010).
Antharam, V. et al. High field magnetic resonance microscopy of the human hippocampus in Alzheimer's disease: quantitative imaging and correlation with iron. Neuroimage 59, 1249–1260 (2012).
Raven, E. P., Lu, P. H., Tishler, T. A., Heydari, P. & Bartzokis, G. Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer's disease detected in vivo with magnetic resonance imaging. J. Alzheimers Dis. 37, 127–136 (2013).
Hametner, S. et al. Iron and neurodegeneration in the multiple sclerosis brain. Ann. Neurol. 74, 848–861 (2013).
Faux, N. G. et al. An anemia of Alzheimer's disease. Mol. Psychiatr. 19, 1227–1234 (2014).
Pichler, I. et al. Serum iron levels and the risk of parkinson disease: a Mendelian randomization study. PLoS Med. 10, e1001462 (2013).
Ali-Rahmani, F., Schengrund, C. L. & Connor, J. R. HFE gene variants, iron, and lipids: a novel connection in Alzheimer's disease. Front. Pharmacol. 5, 165 (2014).
de Lau, L. M. & Breteler, M. M. Epidemiology of Parkinson's disease. Lancet Neurol. 5, 525–535 (2006).
Zhu, X., Raina, A. K., Perry, G. & Smith, M. A. Alzheimer's disease: the two-hit hypothesis. Lancet Neurol. 3, 219–226 (2004).
Doraiswamy, P. M. & Finefrock, A. E. Metals in our minds: therapeutic implications for neurodegenerative disorders. Lancet Neurol. 3, 431–434 (2004).
Devos, D. et al. Targeting chelatable iron as a therapeutic modality in Parkinson's disease. Antioxid. Redox Signal. 21, 195–210 (2014).
Kaur, D. et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron 37, 899–909 (2003).
Kaur, D. et al. Increased murine neonatal iron intake results in Parkinson-like neurodegeneration with age. Neurobiol. Aging 28, 907–913 (2007).
Fernandez, L. L. et al. Effects of increased iron intake during the neonatal period on the brain of adult AβPP/PS1 transgenic mice. J. Alzheimers Dis. 19, 1069–1080 (2010).
Becerril-Ortega, J., Bordji, K., Fréret, T., Rush, T. & Buisson, A. Iron overload accelerates neuronal amyloid-β production and cognitive impairment in transgenic mice model of Alzheimer's disease. Neurobiol. Aging 35, 2288–2301 (2014).
Erikson, K. M., Pinero, D. J., Connor, J. R. & Beard, J. L. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J. Nutr. 127, 2030–2038 (1997).
Powers, K. M. et al. Parkinson's disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology 60, 1761–1766 (2003).
Benarroch, E. E. Brain iron homeostasis and neurodegenerative disease. Neurology 72, 1436–1440 (2009).
Morris, C. M. et al. Brain iron homeostasis. J. Inorg. Biochem. 47, 257–265 (1992).
Zecca, L. et al. The role of iron and copper molecules in the neuronal vulnerability of locus coeruleus and substantia nigra during aging. Proc. Natl Acad. Sci. USA 101, 9843–9848 (2004).
Hare, D. J. et al. An iron–dopamine index predicts risk of parkinsonian neurodegeneration in the substantia nigra pars compacta. Chem. Sci. 5, 2160–2169 (2014).
Hunt, J. R., Zito, C. A. & Johnson, L. K. Body iron excretion by healthy men and women. Am. J. Clin. Nutr. 89, 1792–1798 (2009).
Arora, M. & Austin, C. Teeth as a biomarker of past chemical exposure. Curr. Opin. Pediatr. 25, 261–267 (2013).
Hare, D., Austin, C., Doble, P. & Arora, M. Elemental bio-imaging of trace elements in teeth using laser ablation-inductively coupled plasma-mass spectrometry. J. Dent. 39, 397–403 (2011).
Gunier, R. B. et al. Determinants of manganese in prenatal dentin of shed teeth from CHAMACOS children living in an agricultural community. Environ. Sci. Technol. 47, 11249–11257 (2013).
Arora, M., Hare, D., Austin, C., Smith, D. R. & Doble, P. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci. Total Environ. 409, 1315–1319 (2011).
Austin, C. et al. Barium distributions in teeth reveal early-life dietary transitions in primates. Nature 498, 216–219 (2013).
Bauminger, E., Ofer, S., Gedalia, I., Horowitz, G. & Mayer, I. Iron uptake by teeth and bones: a Mossbauer effect study. Calcif. Tissue Int. 37, 386–389 (1985).
Garfunkel, A., Kantzuker, M., Gedalia, I. & Chevion, M. Iron concentration in teeth of patients with and without beta-thalassaemia major. Arch. Oral Biol. 24, 829–831 (1979).
Ash, M. M. & Nelson, S. J. Wheeler's Dental Anatomy, Physiology and Occlusion 8th edn (WB Saunders, 2003).
Fildes, V. Breasts, Bottles and Babies—A History of Infant Feeding (Edinburgh University Press, 1986).
Bermejo, P. et al. Speciation of iron in breast milk and infant formulas whey by size exclusion chromatography–high performance liquid chromatography and electrothermal atomic absorption spectrometry. Talanta 50, 1211–1222 (2000).
Björklund, K. L. et al. Metals and trace element concentrations in breast milk of first time healthy mothers: a biological monitoring study. Environ. Health 11, 92 (2012).
Ejezie, F. E., Nwagha, U. I., Ikekpeazu, E., Ozoemena, O. & Onwusi, E. Assessment of iron content of breast milk in preterm and term mothers in Enugu urban. Ann. Med. Health Sci. Res. 1, 85–90 (2011).
Krachler, M., Prohaska, T., Koellensperger, G., Rossipal, E. & Stingeder, G. Concentrations of selected trace elements in human milk and in infant formulas determined by magnetic sector field inductively coupled plasma-mass spectrometry. Biol. Trace Elem. Res. 76, 97–112 (2000).
Leotsinidis, M., Alexopoulos, A. & Kostopoulou-Farri, E. Toxic and essential trace elements in human milk from Greek lactating women: association with dietary habits and other factors. Chemosphere 61, 238–247 (2005).
Maru, M., Birhanu, T. & Tessema, D. A. Calcium, magnesium, iron, zinc and copper, compositions of human milk from populations with cereal and 'enset' based diets. Ethiop. J. Health Sci. 23, 90–97 (2013).
Mello-Neto, J. et al. Iron supplementation in pregnancy and breastfeeding and iron, copper and zinc status of lactating women from a human milk bank. J. Trop. Pediatr. 59, 140–144 (2013).
Mello-Neto, J. et al. Iron concentrations in breast milk and selected maternal factors of human milk bank donors. J. Hum. Lact. 26, 175–179 (2010).
World Health Organization & International Atomic Energy Agency. Minor and trace elements in breast milk: report of a joint WHO/IAEA collaborative study. World Health Organization Institutional Repository for Information Sharing [online], (1989).
Johnson, M. A., Smith, M. M. & Edmonds, J. T. Copper, iron, zinc, and manganese in dietary supplements, infant formulas, and ready-to-eat breakfast cereals. Am. J. Clin. Nutr. 67 (5 Suppl.), 1035S–1040S (1998).
Ljung, K., Palm, B., Grandér, M. & Vahter, M. High concentrations of essential and toxic elements in infant formula and infant foods—a matter of concern. Food Chem. 127, 943–951 (2011).
Saarinen, U. M. & Siimes, M. A. Iron absorption from infant milk formula and the optimal level of iron supplementation. Acta Paediatr. Scand. 66, 719–722 (1977).
Walter, T., Pino, P., Pizarro, F. & Lozoff, B. Prevention of iron-deficiency anemia: comparison of high- and low-iron formulas in term healthy infants after six months of life. J. Pediatr. 132, 635–640 (1998).
Chemizmu, K. & Fentona, R. Fenton reaction—controversy concerning the chemistry. Ecol. Chem. Eng. S. 16, 347–358 (2009).
Bartzokis, G. et al. Brain ferritin iron may influence age- and gender-related risks of neurodegeneration. Neurobiol. Aging 28, 414–423 (2007).
Schipper, H. M. Heme oxygenase-1: role in brain aging and neurodegeneration. Exp. Gerontol. 35, 821–830 (2000).
Schipper, H. M. Brain iron deposition and the free radical–mitochondrial theory of ageing. Ageing Res. Rev. 3, 265–301 (2004).
Chen, J. H., Singh, N., Tay, H. & Walczyk, T. Imbalance of iron influx and efflux causes brain iron accumulation over time in the healthy adult rat. Metallomics 6, 1417–1426 (2014).
Kaplan, J. Strategy and tactics in the evolution of iron acquisition. Semin. Hematol. 39, 219–226 (2002).
Quinn, E. A. Too much of a good thing: evolutionary perspectives on infant formula fortification in the United States and its effects on infant health. Am. J. Hum. Biol. 26, 10–17 (2014).
Williams, R. J. Iron in evolution. FEBS Lett. 586, 479–484 (2012).
Grandjean, P. & Landrigan, P. J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 13, 330–338 (2014).
Engelhardt, B. & Liebner, S. Novel insights into the development and maintenance of the blood–brain barrier. Cell Tissue Res. 355, 687–699 (2014).
Abbott, N. J., Patabendige, A. A., Dolman, D. E., Yusof, S. R. & Begley, D. J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 37, 13–25 (2010).
Jones, H. C., Keep, R. F. & Butt, A. M. The development of ion regulation at the blood–brain barrier. Prog. Brain Res. 91, 123–131 (1992).
Xu, J. & Ling, E. A. Studies of the ultrastructure and permeability of the blood–brain barrier in the developing corpus callosum in postnatal rat brain using electron dense tracers. J. Anat. 184, 227–237 (1994).
Morgan, E. H. & Moos, T. Mechanism and developmental changes in iron transport across the blood–brain barrier. Dev. Neurosci. 24, 106–113 (2002).
Taylor, E. M. & Morgan, E. H. Developmental changes in transferrin and iron uptake by the brain in the rat. Brain Res. Dev. Brain. Res. 55, 35–42 (1990).
Dani, C. et al. Effect of blood transfusions on oxidative stress in preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 89, F408–F411 (2004).
McCarthy, R. C. & Kosman, D. J. Iron transport across the blood–brain barrier: development, neurovascular regulation and cerebral amyloid angiopathy. Cell Mol. Life Sci. 72, 709–727 (2015).
Wu, L. J. et al. Expression of the iron transporter ferroportin in synaptic vesicles and the blood–brain barrier. Brain Res. 1001, 108–117 (2004).
Moos, T. & Rosengren Nielsen, T. Ferroportin in the postnatal rat brain: implications for axonal transport and neuronal export of iron. Semin. Pediatr. Neurol. 13, 149–157 (2006).
Simpson, I. A. et al. A novel model for brain iron uptake: introducing the concept of regulation. J. Cereb. Blood Flow Metab. 35, 48–57 (2015).
Bradbury, M. W. Transport of iron in the blood–brain–cerebrospinal fluid system. J. Neurochem. 69, 443–454 (1997).
Moos, T. & Morgan, E. H. Kinetics and distribution of [59Fe–125I]transferrin injected into the ventricular system of the rat. Brain Res. 790, 115–128 (1998).
Chen, J. H., Shahnavas, S., Singh, N., Ong, W. Y. & Walczyk, T. Stable iron isotope tracing reveals significant brain iron uptake in adult rats. Metallomics 5, 167–173 (2013).
Maynard, C. J. et al. Overexpression of Alzheimer's disease amyloid-β opposes the age-dependent elevations of brain copper and iron. J. Biol. Chem. 277, 44670–44676 (2002).
Bilgic, B., Pfefferbaum, A., Rohlfing, T., Sullivan, E. V. & Adalsteinsson, E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. Neuroimage 59, 2625–2635 (2012).
Acknowledgements
The authors' research was supported by a University of Technology, Sydney Chancellor's Postdoctoral Fellowship to D.J.H.; a National Institute of Environmental Health Sciences grant (DP2ES025453—National Institute of Health Director's New Innovator Award; R00ES019597) to M.A.; a Michael J. Fox Foundation for Parkinson's Research grant to D.I.F.; Australian Research Council Linkage Project grants (LP100200254, LP120200081) to D.J.H. and P.A.D.; and Australian National Health and Medical Research Council grants to D.I.F. (APP1043992, APP1044542) and to A.I.B (APP1002222, GNT1037234, APP1044542).
Author information
Authors and Affiliations
Contributions
D.J.H. and M.A. researched the data for and wrote the article. N.I.J., D.I.F., P.A.D. and A.I.B. contributed to discussion of the content and the reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.I.F. is a paid consultant to and a shareholder of Prana Biotechnology. A.I.B. is a shareholder of Prana Biotechnology, Mesoblast, Cogstate, Brighton and Eucalyptus, and is a paid consultant for Collaborative Medicinal Discovery and Brighton. D.J.H., M.A., N.L.J. and P.A.D. declare no competing interests.
Rights and permissions
About this article
Cite this article
Hare, D., Arora, M., Jenkins, N. et al. Is early-life iron exposure critical in neurodegeneration?. Nat Rev Neurol 11, 536–544 (2015). https://doi.org/10.1038/nrneurol.2015.100
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2015.100
This article is cited by
-
A high fat diet potentiates neonatal iron overload-induced memory impairments in rats
European Journal of Nutrition (2024)
-
Perturbed iron biology in the prefrontal cortex of people with schizophrenia
Molecular Psychiatry (2023)
-
Relevance of biometals during neuronal differentiation and myelination: in vitro and in vivo studies
BioMetals (2022)
-
Implication of ferroptosis in aging
Cell Death Discovery (2021)
-
Effects of lipoic acid supplementation on age- and iron-induced memory impairment, mitochondrial DNA damage and antioxidant responses
European Journal of Nutrition (2021)