Key Points
-
Abnormal functional and structural connectivity are candidate biomarkers for Alzheimer disease (AD) and other neurodegenerative diseases
-
The topography of abnormal functional and structural connectivity maps onto the clinical phenotype, and its severity correlates with clinical disease severity in AD and frontotemporal dementia
-
Structural—but, as yet, not functional—connectivity signatures of neurodegenerative diseases with a primary motor phenotype (for example, amyotrophic lateral sclerosis, Parkinson disease and Huntington disease) have been consistently identified
-
Functional connectivity signatures are related to specific molecular pathology in preclinical AD, and could serve as early disease markers
Abstract
Functional and structural connectivity measures, as assessed by means of functional and diffusion MRI, are emerging as potential intermediate biomarkers for Alzheimer disease (AD) and other disorders. This Review aims to summarize current evidence that connectivity biomarkers are associated with upstream and downstream disease processes (molecular pathology and clinical symptoms, respectively) in the major neurodegenerative diseases. The vast majority of studies have addressed functional and structural connectivity correlates of clinical phenotypes, confirming the predictable correlation with topography and disease severity in AD and frontotemporal dementia. In neurodegenerative diseases with motor symptoms, structural—but, to date, not functional—connectivity has been consistently found to be associated with clinical phenotype and disease severity. In the latest studies, the focus has moved towards the investigation of connectivity correlates of molecular pathology. Studies in cognitively healthy individuals with brain amyloidosis or genetic risk factors for AD have shown functional connectivity abnormalities in preclinical disease stages that are reminiscent of abnormalities observed in symptomatic AD. This shift in approach is promising, and may aid identification of early disease markers, establish a paradigm for other neurodegenerative disorders, shed light on the molecular neurobiology of connectivity disruption and, ultimately, clarify the pathophysiology of neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Taylor, J. P., Hardy, J. & Fischbeck, K. H. Toxic proteins in neurodegenerative disease. Science 296, 1991–1995 (2002).
Villemagne, V. L. et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 12, 357–367 (2013).
Frisoni, G. B., Fox, N. C., Jack, C. R. Jr, Scheltens, P. & Thompson, P. M. The clinical use of structural MRI in Alzheimer disease. Nat. Rev. Neurol. 6, 67–77 (2010).
Whitwell, J. L. & Josephs, K. A. Neuroimaging in frontotemporal lobar degeneration—predicting molecular pathology. Nat. Rev. Neurol. 8, 131–142 (2012).
Seeley, W. W., Crawford, R. K., Zhou, J., Miller, B. L. & Greicius, M. D. Neurodegenerative diseases target large-scale human brain networks. Neuron 62, 42–52 (2009).
Raj, A., Kuceyeski, A. & Weiner, M. A network diffusion model of disease progression in dementia. Neuron 73, 1204–1215 (2012).
Moeller, J. R., Strother, S. C., Sidtism, J. J. & Rottenberg, D. A. Scaled subprofile model: a statistical approach to the analysis of functional patterns in positron emission tomographic data. J. Cereb. Blood Flow Metab. 7, 649–658 (1987).
Horwitz, B. Simulating functional interactions in the brain: a model for examining correlations between regonal cerebral metabolic rates. Int. J. Biomed. Comput. 26, 149–170 (1990).
Friston, K. J. Functional and effective connectivity in neuroimaging: a synthesis. Hum. Brain Mapp. 2, 56–78 (1994).
Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541 (1995).
Greicius, M. D. et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry 62, 429–437 (2007).
Greicius, M. D., Srivastava, G., Reiss, A. L. & Menon, V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl Acad. Sci. USA 101, 4637–4642 (2004).
Sorg, C. et al. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc. Natl Acad. Sci. USA 104, 18760–18765 (2007).
Damoiseaux, J. S. et al. Consistent resting-state networks across healthy subjects. Proc. Natl Acad. Sci. USA. 103, 13848–13853 (2006).
Chen, S. et al. Group independent component analysis reveals consistent resting-state networks across multiple sessions. Brain Res. 1239, 141–151 (2008).
Goldman, R. I., Stern, J. M., Engel, J. Jr & Cohen, M. S. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 13, 2487–2492 (2002).
Raichle, M. E. et al. A default mode of brain function. Proc. Natl Acad. Sci. USA 98, 676–682 (2001).
Biswal, B. B. et al. Toward discovery science of human brain function. Proc. Natl Acad. Sci. USA 107, 4734–4739 (2010).
Fukunaga, M. et al. Metabolic origin of BOLD signal fluctuations in the absence of stimuli. J. Cereb. Blood Flow Metab. 28, 1377–1387 (2008).
Vincent, J. L. et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature 447, 83–86 (2007).
Lu, H. et al. Rat brains also have a default mode network. Proc. Natl Acad. Sci. USA 109, 3979–3984 (2012).
Leech, R. & Sharp, D. J. The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32 (2014).
Utevsky, A. V., Smith, D. V. & Huettel, S. A. Precuneus is a functional core of the default-mode network. J. Neurosci. 34, 932–940 (2014).
Seeley, W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007).
Smith, S. M. et al. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl Acad. Sci. USA 106, 13040–13045 (2009).
Zhang, D. et al. Intrinsic functional relations between human cerebral cortex and thalamus. J. Neurophysiol. 100, 1740–1748 (2008).
Alexander, A. L., Lee, J. E., Lazar, M. & Field, A. S. Diffusion tensor imaging of the brain. Neurotherapeutics 4, 316–329 (2007).
Catani, M. & Thiebaut de Schotten, M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44, 1105–1132 (2008).
Petrella, J. R., Sheldon, F. C., Prince, S. E., Calhoun, V. D. & Doraiswamy, P. M. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology 76, 511–517 (2011).
Agosta, F. et al. Resting state fMRI in Alzheimer's disease: beyond the default mode network. Neurobiol. Aging 33, 1564–1578 (2012).
Binnewijzend, M. A. et al. Resting-state fMRI changes in Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging 33, 2018–2028 (2012).
Brier, M. R. et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J. Neurosci. 32, 8890–8899 (2012).
Zhang, H. Y. et al. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology 256, 598–606 (2010).
Zamboni, G. et al. Resting functional connectivity reveals residual functional activity in Alzheimer's disease. Biol. Psychiatry 74, 375–383 (2013).
Zhou, J. et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 133, 1352–1367 (2010).
Damoiseaux, J. S., Prater, K. E., Miller, B. L. & Greicius, M. D. Functional connectivity tracks clinical deterioration in Alzheimer's disease. Neurobiol. Aging 33, 828.e19–828.e30 (2012).
Pievani, M. et al. Assessment of white matter tract damage in mild cognitive impairment and Alzheimer's disease. Hum. Brain Mapp. 31, 1862–1875 (2010).
Huang, H. et al. Distinctive disruption patterns of white matter tracts in Alzheimer's disease with full diffusion tensor characterization. Neurobiol. Aging 33, 2029–2045 (2012).
Acosta-Cabronero, J., Alley, S., Williams, G. B., Pengas, G. & Nestor, P. J. Diffusion tensor metrics as biomarkers in Alzheimer's disease. PLoS ONE 7, e49072 (2012).
Douaud, G. et al. Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to Alzheimer's disease. J. Neurosci. 33, 2147–2155 (2013).
Zhang, Y. et al. White matter damage in frontotemporal dementia and Alzheimer's disease measured by diffusion MRI. Brain 132, 2579–2592 (2009).
Filippi, M. et al. Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex 49, 2389–2401 (2013).
Song, J. et al. Aberrant functional organization within and between resting-state networks in AD. PLoS ONE 8, e63727 (2013).
Farb, N. A. et al. Abnormal network connectivity in frontotemporal dementia: evidence for prefrontal isolation. Cortex 49, 1856–1873 (2013).
Borroni, B. et al. Evidence of white matter changes on diffusion tensor imaging in frontotemporal dementia. Arch. Neurol. 64, 246–251 (2007).
Hornberger, M., Geng, J. & Hodges, J. R. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain 134, 2502–2512 (2011).
Mahoney, C. J. et al. Profiles of white matter tract pathology in frontotemporal dementia. Hum. Brain Mapp. 35, 4163–4179 (2014).
Tovar-Moll, F. et al. White matter tract damage in the behavioral variant of frontotemporal and corticobasal dementia syndromes. PLoS ONE 9, e102656 (2014).
Whitwell, J. L. et al. Altered functional connectivity in asymptomatic MAPT subjects: a comparison to bvFTD. Neurology 77, 866–874 (2011).
Gardner, R. C. et al. Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann. Neurol. 73, 603–616 (2013).
Whitwell, J. L. et al. Disrupted thalamocortical connectivity in PSP: a resting-state fMRI, DTI, and VBM study. Parkinsonism Relat. Disord. 17, 599–605 (2011).
Boxer, A. L. et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch. Neurol. 63, 81–86 (2006).
Canu, E. et al. Diffusion tensor magnetic resonance imaging tractography in progressive supranuclear palsy. Mov. Disord. 26, 1752–1755 (2011).
Whitwell, J. L. et al. Clinical correlates of white matter tract degeneration in progressive supranuclear palsy. Arch. Neurol. 68, 753–760 (2011).
Sajjadi, S. A. et al. Diffusion tensor magnetic resonance imaging for single subject diagnosis in neurodegenerative diseases. Brain 136, 2253–2261 (2013).
Mohammadi, B. et al. Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp. Neurol. 217, 147–153 (2009).
Tedeschi, G. et al. Interaction between aging and neurodegeneration in amyotrophic lateral sclerosis. Neurobiol. Aging 33, 886–898 (2012).
Zhou, F. et al. Altered motor network functional connectivity in amyotrophic lateral sclerosis: a resting-state functional magnetic resonance imaging study. Neuroreport 24, 657–662 (2013).
Zhou, F. et al. Alterations in regional functional coherence within the sensory-motor network in amyotrophic lateral sclerosis. Neurosci. Lett. 558, 192–196 (2014).
Verstraete, E. et al. Motor network degeneration in amyotrophic lateral sclerosis: a structural and functional connectivity study. PLoS ONE 5, e13664 (2010).
Douaud, G., Filippini, N., Knight, S., Talbot, K. & Turner, M. R. Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis. Brain 134, 3470–3479 (2011).
Verstraete, E., Veldink, J. H., Mandl, R. C., van den Berg, L. H. & van den Heuvel, M. P. Impaired structural motor connectome in amyotrophic lateral sclerosis. PLoS ONE 6, e24239 (2011).
Agosta, F. et al. Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor MR imaging tractography study. AJNR Am. J. Neuroradiol. 31, 1457–1461 (2010).
Rose, S. et al. Direct evidence of intra- and interhemispheric corticomotor network degeneration in amyotrophic lateral sclerosis: an automated MRI structural connectivity study. Neuroimage 59, 2661–2669 (2012).
Bede, P. et al. Multiparametric MRI study of ALS stratified for the C9orf72 genotype. Neurology 81, 361–369 (2013).
Trojsi, F. et al. Motor and extramotor neurodegeneration in amyotrophic lateral sclerosis: a 3 T high angular resolution diffusion imaging (HARDI) study. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, 553–561 (2013).
Thivard, L. et al. Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: relationships with motor disability. J. Neurol. Neurosurg. Psychiatry 78, 889–892 (2007).
Zhang, J. et al. Regional alterations in cortical thickness and white matter integrity in amyotrophic lateral sclerosis. J. Neurol. 261, 412–421 (2014).
Stanton, B. R. et al. Diffusion tensor imaging in sporadic and familial (D90A SOD1) forms of amyotrophic lateral sclerosis. Arch. Neurol. 66, 109–115 (2009).
Blain, C. R. et al. Differential corticospinal tract degeneration in homozygous 'D90A' SOD-1 ALS and sporadic ALS. J. Neurol. Neurosurg. Psychiatry 82, 843–849 (2011).
Crespi, C. et al. Microstructural white matter correlates of emotion recognition impairment in amyotrophic lateral sclerosis. Cortex 53, 1–8 (2014).
Sage, C. A., Peeters, R. R., Görner, A., Robberecht, W. & Sunaert, S. Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage 34, 486–499 (2007).
Keil, C. et al. Longitudinal diffusion tensor imaging in amyotrophic lateral sclerosis. BMC Neurosci. 13, 141 (2012).
Senda, J. et al. Progressive and widespread brain damage in ALS: MRI voxel-based morphometry and diffusion tensor imaging study. Amyotroph. Lateral Scler. 12, 59–69 (2011).
Verstraete, E., Veldink, J. H., van den Berg, L. H. & van den Heuvel, M. P. Structural brain network imaging shows expanding disconnection of the motor system in amyotrophic lateral sclerosis. Hum. Brain Mapp. 35, 1351–1361 (2014).
Agosta, F. et al. Sensorimotor functional connectivity changes in amyotrophic lateral sclerosis. Cereb. Cortex 21, 2291–2298 (2011).
Schmidt, R. et al. Correlation between structural and functional connectivity impairment in amyotrophic lateral sclerosis. Hum. Brain Mapp. 35, 4386–4395 (2014).
Agosta, F. et al. Divergent brain network connectivity in amyotrophic lateral sclerosis. Neurobiol. Aging 34, 419–427 (2013).
Esposito, F. et al. Rhythm-specific modulation of the sensorimotor network in drug-naive patients with Parkinson's disease by levodopa. Brain 136, 710–725 (2013).
Luo, C. et al. Reduced functional connectivity in early-stage drug-naive Parkinson's disease: a resting-state fMRI study. Neurobiol. Aging 35, 431–441 (2014).
Kwak, Y. et al. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front. Syst. Neurosci. 4, 143 (2010).
Yu, R., Liu, B., Wang, L., Chen, J. & Liu, X. Enhanced functional connectivity between putamen and supplementary motor area in Parkinson's disease patients. PLoS ONE 8, e59717 (2013).
Wu, T. et al. Changes of functional connectivity of the motor network in the resting state in Parkinson's disease. Neurosci. Lett. 460, 6–10 (2009).
Helmich, R. C. et al. I. Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb. Cortex 20, 1175–1186 (2010).
Choe, I. H., Yeo, S., Chung, K. C., Kim, S. H. & Lim, S. Decreased and increased cerebral regional homogeneity in early Parkinson's disease. Brain Res. 1527, 230–237 (2013).
Hacker, C. D., Perlmutter, J. S., Criswell, S. R., Ances, B. M. & Snyder, A. Z. Resting state functional connectivity of the striatum in Parkinson's disease. Brain 135, 3699–3711 (2012).
Göttlich, M. et al. Altered resting state brain networks in Parkinson's disease. PLoS ONE 8, e77336 (2013).
Agosta, F. et al. The topography of brain damage at different stages of Parkinson's disease. Hum. Brain Mapp. 34, 2798–2807 (2013).
Melzer, T. R. et al. White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology 80, 1841–1849 (2013).
Tessitore, A. et al. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 79, 2226–2232 (2012).
Dumas, E. M. et al. Reduced functional brain connectivity prior to and after disease onset in Huntington's disease. Neuroimage Clin. 2, 377–384 (2013).
Poudel, G. R. et al. Abnormal synchrony of resting state networks in premanifest and symptomatic Huntington disease: the IMAGE-HD study. J. Psychiatry Neurosci. 39, 87–96 (2014).
Werner, C. J. et al. Altered resting-state connectivity in Huntington's disease. Hum. Brain Mapp. 35, 2582–2593 (2014).
Quarantelli, M. et al. Default-mode network changes in Huntington's disease: an integrated MRI study of functional connectivity and morphometry. PLoS ONE 8, e72159 (2013).
Grahn, J. A., Parkinson, J. A. & Owen, A. M. The role of the basal ganglia in learning and memory: neuropsychological studies. Behav. Brain Res. 199, 53–60 (2009).
Novak, M. J. et al. White matter integrity in premanifest and early Huntington's disease is related to caudate loss and disease progression. Cortex 52, 98–112 (2014).
Della Nave, R. et al. Regional distribution and clinical correlates of white matter structural damage in Huntington disease: a tract-based spatial statistics study. AJNR Am. J. Neuroradiol. 31, 1675–1681 (2010).
Bohanna, I., Georgiou-Karistianis, N. & Egan, G. F. Connectivity-based segmentation of the striatum in Huntington's disease: vulnerability of motor pathways. Neurobiol. Dis. 42, 475–481 (2011).
Rosas, H. D. et al. Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical “disconnection”. Neuroimage 49, 2995–3004 (2010).
Bohanna, I. et al. Diffusion tensor imaging in Huntington's disease reveals distinct patterns of white matter degeneration associated with motor and cognitive deficits. Brain Imaging Behav. 5, 171–180 (2011).
Lehmann, M. et al. Intrinsic connectivity networks in healthy subjects explain clinical variability in Alzheimer's disease. Proc. Natl Acad. Sci. USA 110, 11606–11611 (2013).
Gour, N. et al. Functional connectivity changes differ in early and late-onset Alzheimer's disease. Hum. Brain Mapp. 35, 2978–2994 (2014).
Canu, E. et al. White matter microstructural damage in Alzheimer's disease at different ages of onset. Neurobiol. Aging 34, 2331–2340 (2013).
Migliaccio, R. et al. Brain networks in posterior cortical atrophy: a single case tractography study and literature review. Cortex 48, 1298–1309 (2012).
Duning, T. et al. Pattern and progression of white-matter changes in a case of posterior cortical atrophy using diffusion tensor imaging. J. Neurol. Neurosurg. Psychiatry 80, 432–436 (2009).
Agosta, F. et al. White matter damage in frontotemporal lobar degeneration spectrum. Cereb. Cortex 22, 2705–2714 (2012).
Zhang, Y. et al. MRI signatures of brain macrostructural atrophy and microstructural degradation in frontotemporal lobar degeneration subtypes. J. Alzheimers Dis. 33, 431–444 (2013).
Whitwell, J. L. et al. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology 74, 1279–1287 (2010).
Lillo, P. et al. Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS ONE 7, e43993 (2012).
Rajagopalan, V., Yue, G. H. & Pioro, E. P. Brain white matter diffusion tensor metrics from clinical 1.5 T MRI distinguish between ALS phenotypes. J. Neurol. 260, 2532–2540 (2013).
Agosta, F. et al. Diffusion tensor MRI contributes to differentiate Richardson's syndrome from PSP-parkinsonism. Neurobiol. Aging 33, 2817–2826 (2012).
Vos, S. J. et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 12, 957–965 (2013).
Hedden, T. et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J. Neurosci. 29, 12686–12694 (2009).
Sheline, Y. I. et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol. Psychiatry 67, 584–587 (2010).
Drzezga, A. et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain 134, 1635–1646 (2011).
Wang, L. et al. Cerebrospinal fluid Aβ42, phosphorylated Tau181, and resting-state functional connectivity. JAMA Neurol. 70, 1242–1248 (2013).
Brier, M. R. et al. Functional connectivity and graph theory in preclinical Alzheimer's disease. Neurobiol. Aging 35, 757–768 (2014).
Strittmatter, W. J. et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl Acad. Sci. USA 90, 1977–1981 (1993).
Machulda, M. M. et al. Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch. Neurol. 68, 1131–1136 (2011).
Patel, K. T. et al. Default mode network activity and white matter integrity in healthy middle-aged ApoE4 carriers. Brain Imaging Behav. 7, 60–67 (2013).
Brown, J. A. et al. Brain network local interconnectivity loss in aging APOE-4 allele carriers. Proc. Natl Acad. Sci. USA 108, 20760–20765 (2011).
Honea, R. A., Vidoni, E., Harsha, A. & Burns, J. M. Impact of APOE on the healthy aging brain: a voxel-based MRI and DTI study. J. Alzheimers Dis. 18, 553–564 (2009).
Chiang, G. C., Zhan, W., Schuff, N. & Weiner, M. W. White matter alterations in cognitively normal apoE ε2 carriers: insight into Alzheimer resistance? AJNR Am. J. Neuroradiol. 33, 1392–1397 (2012).
Sheline, Y. I. et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. J. Neurosci. 30, 17035–17040 (2010).
Filippini, N. et al. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl Acad. Sci. USA 106, 7209–7214 (2009).
Dennis, N. A. et al. Temporal lobe functional activity and connectivity in young adult APOE e4 carriers. Alzheimers Dement. 6, 303–311 (2010).
Dowell, N. G. et al. MRI of carriers of the apolipoprotein E e4 allele-evidence for structural differences in normal-appearing brain tissue in e4+ relative to e4− young adults. NMR Biomed. 26, 674–682 (2013).
O'Dwyer, L. et al. White matter differences between healthy young ApoE4 carriers and non-carriers identified with tractography and support vector machines. PLoS ONE 7, e36024 (2012).
Dean, D. C. et al. Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol. 71, 11–22 (2014).
McMillan, C. T. et al. White matter imaging helps dissociate tau from TDP-43 in frontotemporal lobar degeneration. J. Neurol. Neurosurg. Psychiatry 84, 949–955 (2013).
McMillan, C. T. et al. Genetic and neuroanatomic associations in sporadic frontotemporal lobar degeneration. Neurobiol. Aging 35, 1473–1482 (2014).
Premi, E. et al. Effect of TMEM106B polymorphism on functional network connectivity in asymptomatic GRN mutation carriers. JAMA Neurol. 71, 216–221 (2014).
Sala-Llonch, R. et al. Evolving brain functional abnormalities in PSEN1 mutation carriers: a resting and visual encoding fMRI study. J. Alzheimers Dis. 36, 165–175 (2013).
Chhatwal, J. P. et al. Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology 81, 736–744 (2013).
Borroni, B. et al. Granulin mutation drives brain damage and reorganization from preclinical to symptomatic FTLD. Neurobiol. Aging 33, 2506–2520 (2012).
Dopper, E. G. et al. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology 80, 814–823 (2013).
Pievani, M. et al. Pattern of structural and functional brain abnormalities in asymptomatic granulin mutation carriers. Alzheimers Dement. http://dx.doi.org/10.1016/j.jalz.2013.09.009.
Wu, T. et al. Preclinical and clinical neural network changes in SCA2 parkinsonism. Parkinsonism Relat. Disord. 19, 158–164 (2013).
Unschuld, P. G. et al. Impaired cortico-striatal functional connectivity in prodromal Huntington's disease. Neurosci. Lett. 514, 204–209 (2012).
Ringman, J. M. et al. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer's disease mutations. Brain 130, 1767–1776 (2007).
Ryan, N. S. et al. Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer's disease. Brain 136, 1399–1414 (2013).
Fortea, J. et al. Increased cortical thickness and caudate volume precede atrophy in PSEN1 mutation carriers. J. Alzheimers Dis. 22, 909–922 (2010).
Borroni, B. et al. Brain magnetic resonance imaging structural changes in a pedigree of asymptomatic progranulin mutation carriers. Rejuvenation Res. 11, 585–595 (2008).
Ng, M. C. et al. Abnormal diffusion tensor in nonsymptomatic familial amyotrophic lateral sclerosis with a causative superoxide dismutase 1 mutation. J. Magn. Reson. Imaging 27, 8–13 (2008).
Dumas, E. M. et al. Early changes in white matter pathways of the sensorimotor cortex in premanifest Huntington's disease. Hum. Brain Mapp. 33, 203–212 (2012).
Rosas, H. D. et al. Diffusion tensor imaging in presymptomatic and early Huntington's disease: Selective white matter pathology and its relationship to clinical measures. Mov. Disord. 21, 1317–1325 (2006).
Warren, J. D., Rohrer, J. D. & Hardy, J. Disintegrating brain networks: from syndromes to molecular nexopathies. Neuron 73, 1060–1062 (2012).
Dian Observational Study. Dominantly Inherited Alzheimer Network [online], (2013).
GENFI: the Genetic Frontotemporal Dementia Initiative. UCL [online], (2014).
Damoiseaux, J. S. & Greicius, M. D. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Struct. Funct. 213, 525–533 (2009).
Honey, C. J. et al. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl Acad. Sci. USA 106, 2035–2040 (2009).
Hagmann, P. et al. MR connectomics: principles and challenges. J. Neurosci. Methods 194, 34–45 (2010).
van den Heuvel, M. P. & Sporns, O. An anatomical substrate for integration among functional networks in human cortex. J. Neurosci. 33, 14489–14500 (2013).
Zhou, J., Gennatas, E. D., Kramer, J. H., Miller, B. L. & Seeley, W. W. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron 73, 1216–1227 (2012).
Bullmore, E. & Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 13, 336–349 (2012).
van den Heuvel, M. P. & Sporns, O. Rich-club organization of the human connectome. J. Neurosci. 31, 15775–15786 (2011).
van den Heuvel, M. P., Kahn, R. S., Goñi, J. & Sporns, O. High-cost, high-capacity backbone for global brain communication. Proc. Natl Acad. Sci. USA 109, 11372–11377 (2012).
Collin, G., Sporns, O., Mandl, R. C. & van den Heuvel, M. P. Structural and functional aspects relating to cost and benefit of rich club organization in the human cerebral cortex. Cereb. Cortex 24, 2258–2267 (2014).
Morrison, J. H. & Hof, P. R. Life and death of neurons in the aging brain. Science 278, 412–419 (1997).
van den Heuvel, M. P. et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry 70, 783–792 (2013).
Hu, W. T. et al. Distinct cerebral perfusion patterns in FTLD and AD. Neurology 75, 881–888 (2010).
Arterial spin labelling Initiative in Dementia (AID). COST: European Cooperation in Science and Technology [online], (2014).
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L. & Petersen, S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154 (2012).
Uludag˘, K. et al. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. Neuroimage 23, 148–155 (2004).
Danielian, L. E., Iwata, N. K., Thomasson, D. M. & Floeter, M. K. Reliability of fiber tracking measurements in diffusion tensor imaging for longitudinal study. Neuroimage 49, 1572–1580 (2010).
Zuo, X. N. et al. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage 49, 2163–2177 (2010).
McMillan, C. T. et al. White matter imaging contributes to the multimodal diagnosis of frontotemporal lobar degeneration. Neurology 78, 1761–1768 (2012).
Acknowledgements
N.F. is funded by the HDH Wills 1965 Charitable Trust. M.P.v.d.H. is supported by a Fellowship from the Brain Center Rudolf Magnus and a VENI grant from the Dutch Council for Research (NWO).
Author information
Authors and Affiliations
Contributions
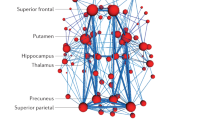
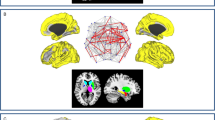
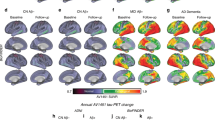
M.P. and G.B.F. developed the architecture of the manuscript. M.P. wrote the initial draft, which was completed, edited and reviewed for important intellectual content by N.F., M.P.v.d.H., S.F.C. and G.B.F. M.P. and G.B.F. prepared Figure 1, Figure 3 and Figure 4, and N.F. prepared Figure 2. All the authors have seen and approved the final version.
Corresponding author
Ethics declarations
Competing interests
G.B.F. has served on the advisory boards for Lilly, BMS, Bayer, Lundbeck, Elan, AstraZeneca, Pfizer, Baxter, Taurx and Wyeth, and has received research support from Wyeth, Lilly and Lundbeck Italia. The other authors declare no competing interests.
Supplementary information
Supplementary Table 1
Technical variability (magnetic field strength and post-processing method) across studies on resting-state functional connectivity MRI (PDF 157 kb)
Rights and permissions
About this article
Cite this article
Pievani, M., Filippini, N., van den Heuvel, M. et al. Brain connectivity in neurodegenerative diseases—from phenotype to proteinopathy. Nat Rev Neurol 10, 620–633 (2014). https://doi.org/10.1038/nrneurol.2014.178
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2014.178
This article is cited by
-
Locating causal hubs of memory consolidation in spontaneous brain network in male mice
Nature Communications (2023)
-
Different MRI structural processing methods do not impact functional connectivity computation
Scientific Reports (2023)
-
Functional brain networks in the evaluation of patients with neurodegenerative disorders
Nature Reviews Neurology (2023)
-
The BrainLat project, a multimodal neuroimaging dataset of neurodegeneration from underrepresented backgrounds
Scientific Data (2023)
-
Connectomics-based resting-state functional network alterations predict suicidality in major depressive disorder
Translational Psychiatry (2023)