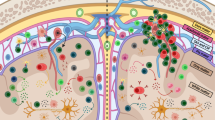
Abstract
Multiple sclerosis (MS) is considered to be a predominantly T-cell-mediated disease, and emerging evidence indicates that dendritic cells have a critical role in the initiation and progression of this debilitating condition. Dendritic cells are specialized antigen-presenting cells that can prime naive T cells and modulate adaptive immune responses. Their powerful biological functions indicate that these cells can be exploited by immunotherapeutic approaches. Therapies that inhibit the immunogenic actions of dendritic cells through the blockade of proinflammatory cytokine production and T cell co-stimulatory pathways are currently being pursued. Furthermore, novel strategies that can regulate dendritic cell development and differentiation and harness the tolerogenic capacity of these cells are also being developed. Here, we evaluate the prospects of these future therapeutic strategies, which focus on dendritic cells and dendritic cell-related targets to treat MS.
Key Points
-
Dendritic cells are necessary for inducing immunity and regulating immune tolerance
-
Potential autoimmune disease treatments that target dendritic cells either inhibit the immunogenic functions of these cells or support their tolerogenic potential
-
Most therapeutic strategies use monoclonal antibodies to target molecules selectively expressed by dendritic cells
-
At least four distinct subtypes of mammalian dendritic cell are known to exist, but their functions and abilities to mobilize T cells during chronic inflammation are not completely understood
-
Dendritic cell-based therapies have been tested in animal models of multiple sclerosis, and a number of these therapies are currently being investigated in other autoimmune diseases, such as rheumatoid arthritis
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ben-Nun, A., Wekerle, H. & Cohen, I. R. The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur. J. Immunol. 11, 195–199 (1981).
McFarland, H. F. & Martin, R. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 8, 913–919 (2007).
Oksenberg, J. R. et al. The genetics of multiple sclerosis: SNPs to pathways to pathogenesis. Nat. Rev. Genet. 9, 516–526 (2008).
Qin, Y. et al. Clonal expansion and somatic hypermutation of VH genes of B cells from cerebrospinal fluid in multiple sclerosis. J. Clin. Invest. 102, 1045–1050 (1998).
Babbe, H. et al. Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J. Exp. Med. 192, 393–404 (2000).
Colombo, M. et al. Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J. Immunol. 164, 2782–2789 (2000).
Obermeier, B. et al. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat. Med. 14, 688–693 (2008).
Meinl, E. Myelin basic protein-specific T lymphocyte repertoire in multiple sclerosis. Complexity of the response and dominance of nested epitopes due to recruitment of multiple T cell clones. J. Clin. Invest. 92, 2633–2643 (1993).
Muraro, P. A. Molecular tracking of antigen-specific T cell clones in neurological immune-mediated disorders. Brain 126, 20–31 (2003).
Skulina, C. Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc. Natl Acad. Sci. USA 101, 2428–2433 (2004).
Steinman, R. M. Dendritic cells: understanding immunogenicity. Eur. J. Immunol. 37, S53–S60 (2007).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Steinman, R. M. & Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007).
Steinman, R. M. Dendritic cells in vivo: a key target for a new vaccine science. Immunity 29, 319–324 (2008).
Steinman, R. M. & Cohn, Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 137, 1142–1162 (1973).
Steinman, R. M. & Cohn, Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J. Exp. Med. 139, 380–397 (1974).
Yang, L. et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat. Biotechnol. 26, 326–334 (2008).
Nchinda, G. et al. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J. Clin. Invest. 118, 1427–1436 (2008).
Liu, Y. J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23, 275–306 (2005).
Wu, L. & Liu, Y. J. Development of dendritic-cell lineages. Immunity 26, 741–750 (2007).
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007).
Liu, K. et al. In vivo analysis of dendritic cell development and homeostasis. Science 324, 392–397 (2009).
Fogg, D. K. et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311, 83–87 (2006).
Liu, K. et al. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 8, 578–583 (2007).
D'Amico, A. & Wu, L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J. Exp. Med. 198, 293–303 (2003).
Karsunky, H. et al. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 198, 305–313 (2003).
Waskow, C. et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat. Immunol. 9, 676–683 (2008).
León, B., López-Bravo, M. & Ardavín, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26, 519–531 (2007).
Shortman, K. & Naik, S. H. Steady-state and inflammatory dendritic-cell development. Nat. Rev. Immunol. 7, 19–30 (2007).
Matyszak, M. K. & Perry, V. H. The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience 74, 599–608 (1996).
Pashenkov, M. & Link, H. Dendritic cells and immune responses in the central nervous system. Trends Immunol. 23, 69–70 (2002).
Serafini, B. et al. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J. Neuropathol. Exp. Neurol. 65, 124–141 (2006).
Lande, R. et al. Plasmacytoid dendritic cells in multiple sclerosis: intracerebral recruitment and impaired maturation in response to interferon-β. J. Neuropathol. Exp. Neurol. 67, 388–401 (2008).
Bulloch, K. et al. CD11c/EYFP transgene illuminates a discrete network of dendritic cells within the embryonic, neonatal, adult, and injured mouse brain. J. Comp. Neurol. 508, 687–710 (2008).
Gottfried-Blackmore, A. et al. Acute in vivo exposure to interferon-γ enables resident brain dendritic cells to become effective antigen presenting cells. Proc. Natl Acad. Sci. USA. 106, 20918–20923 (2009).
Fischer, H. G. & Reichmann, G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J. Immunol. 166, 2717–2726 (2001).
Santambrogio, L. Developmental plasticity of CNS microglia. Proc. Natl Acad. Sci. USA 98, 6295–6300 (2001).
Dittel, B. N. et al. Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J. Immunol. 163, 32–39 (1999).
McMahon, E. J. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat. Med. 11, 335–339 (2005).
Greter, M. et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 11, 328–334 (2005).
Bailey, S. L. et al. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ TH-17 cells in relapsing EAE. Nat. Immunol. 8, 172–180 (2007).
Suter, T. et al. The brain as an immune privileged site: dendritic cells of the central nervous system inhibit T cell activation. Eur. J. Immunol. 33, 2998–3006 (2003).
Khoury, S. J. et al. Mechanisms of acquired thymic tolerance in experimental autoimmune encephalomyelitis: thymic dendritic-enriched cells induce specific peripheral T cell unresponsiveness in vivo. J. Exp. Med. 182, 357–366 (1995).
Karni, A. et al. Innate immunity in multiple sclerosis: myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J. Immunol. 177, 4196–4202 (2006).
Huang, Y. M. et al. Multiple sclerosis is associated with high levels of circulating dendritic cells secreting pro-inflammatory cytokines. J. Neuroimmunol. 99, 82–90 (1999).
Vaknin-Dembinsky, A., Balashov, K. & Weiner, H. L. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J. Immunol. 176, 7768–7774 (2006).
Stasiolek, M. et al. Impaired maturation and altered regulatory function of plasmacytoid dendritic cells in multiple sclerosis. Brain 129, 1293–1305 (2006).
López, C. et al. Altered maturation of circulating dendritic cells in primary progressive MS patients. J. Neuroimmunol. 175, 183–191 (2006).
Schwab, N. et al. An imbalance of two functionally and phenotypically different subsets of plasmacytoid dendritic cells characterizes the dysfunctional immune regulation in multiple sclerosis. J. Immunol. 184, 5368–5374 (2010).
Ramgolam, V. S. et al. IFN-β inhibits human Th17 cell differentiation. J. Immunol. 183, 5418–5427 (2009).
Durelli, L. et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-β. Ann. Neurol. 65, 499–509 (2009).
Axtell, R. C. et al. T helper type 1 and 17 cells determine efficacy of interferon-β in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 16, 406–412 (2010).
Comabella, M. et al. A type I interferon signature in monocytes is associated with poor response to interferon-β in multiple sclerosis. Brain 132, 3353–3365 (2009).
Vieira, P. L. et al. Glatiramer acetate (copolymer-1, copaxone) promotes Th2 cell development and increased IL-10 production through modulation of dendritic cells. J. Immunol. 170, 4483–4488 (2003).
Kim, H. J. et al. Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. J. Immunol. 172, 7144–7153 (2004).
Weber, M. S. et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat. Med. 13, 935–943 (2007).
Burger, D. et al. Glatiramer acetate increases IL-1 receptor antagonist but decreases T cell-induced IL-1β in human monocytes and multiple sclerosis. Proc. Natl Acad. Sci. USA 106, 4355–4359 (2009).
Stüve, O. et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann. Neurol. 59, 743–747 (2006).
del Pilar Martin, M. et al. Decrease in the numbers of dendritic cells and CD4+ T cells in cerebral perivascular spaces due to natalizumab. Arch. Neurol. 65, 1596–1603 (2008).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Matsuki, T. et al. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int. Immunol. 18, 399–407 (2006).
Cannella, B. & Raine, C. S. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann. Neurol. 37, 424–435 (1995).
Okuda, Y. et al. IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glucoprotein 35–55 induced experimental autoimmune encephalomyelitis. J. Neuroimmunol. 101, 188–196 (1999).
Mendel, I. et al. Interleukin-6 functions in autoimmune encephalomyelitis: a study in gene-targeted mice. Eur. J. Immunol. 28, 1727–1737 (1998).
Korn, T. et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA 105, 18460–18465 (2008).
Smolen, J. S. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371, 987–997 (2008).
Windhagen, A. et al. Expression of costimulatory molecules B7–1 (CD80), B7–2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J. Exp. Med. 182, 1985–1996 (1995).
Li, Y. et al. Increased IL-23p19 expression in multiple sclerosis lesions and its induction in microglia. Brain 130, 490–501 (2007).
Becher, B., Durell, B. G. & Noelle, R. J. IL-23 produced by CNS-resident cells controls T cell encephalitogenicity during the effector phase of experimental autoimmune encephalomyelitis. J. Clin. Invest. 112, 1186–1191 (2003).
Cua, D. J., Sherlock, J. & Chen, Y. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Segal, B. M. et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing–remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 7, 796–804 (2008).
Panitch, H. S., Hirsch, R. L., Haley, A. S. & Johnson, K. P. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet 329, 893–895 (1987).
[No authors listed] TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. Neurology 53, 457–465 (1999).
Merad, M. & Manz, M. G. Dendritic cell homeostasis. Blood 113, 3418–3427 (2009).
Hamilton, J. A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544 (2008).
Vremec, D. et al. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur. J. Immunol. 27, 40–44 (1997).
Mildner, A. et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 132, 2487–2500 (2009).
Salomon, B. & Bluestone, J. A. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu. Rev. Immunol. 19, 225–252 (2001).
Bluestone, J. A., St Clair, E. W. & Turka, L. A. CTLA4Ig: bridging the basic immunology with clinical application. Immunity 24, 233–238 (2006).
Dhodapkar, M. V. et al. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J. Exp. Med. 193, 233–238 (2001).
Weiner, H. L. et al. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science 259, 1321–1324 (1993).
Meyer, A. L. et al. Suppression of murine chronic relapsing experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. J. Immunol. 157, 4230–4238 (1996).
Nicholson, L. B. et al. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity 3, 397–405 (1995).
Anderton, S. M. et al. Fine specificity of the myelin-reactive T cell repertoire: implications for TCR antagonism in autoimmunity. J. Immunol. 161, 3357–3364 (1998).
Critchfield, J. M. et al. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science 263, 1139–1143 (1994).
Elliott, E. A. et al. Treatment of experimental encephalomyelitis with a novel chimeric fusion protein of myelin basic protein and proteolipid protein. J. Clin. Invest. 98, 1602–1612 (1996).
McFarland, H. I. et al. Effective antigen-specific immunotherapy in the marmoset model of multiple sclerosis. J. Immunol. 166, 2116–2121 (2001).
Lopez-Diego, R. S. & Weiner, H. L. Novel therapeutic strategies for multiple sclerosis—a multifaceted adversary. Nat. Rev. Drug Discov. 7, 909–925 (2008).
Lutterotti, A., Sospedra, M. & Martin, R. Antigen-specific therapies in MS—Current concepts and novel approaches. J. Neurol. Sci. 274, 18–22 (2008).
Warren, K. G. et al. Intravenous synthetic peptide MBP8298 delayed disease progression in an HLA Class II-defined cohort of patients with progressive multiple sclerosis: results of a 24-month double-blind placebo-controlled clinical trial and 5 years of follow-up treatment. Eur. J. Neurol. 13, 887–895 (2006).
Lobell, A. et al. Vaccination with DNA encoding an immunodominant myelin basic protein peptide targeted to Fc of immunoglobulin G suppresses experimental autoimmune encephalomyelitis. J. Exp. Med. 187, 1543–1548 (1998).
Weissert, R. et al. Protective DNA vaccination against organ-specific autoimmunity is highly specific and discriminates between single amino acid substitutions in the peptide autoantigen. Proc. Natl Acad. Sci. USA 97, 1689–1694 (2000).
Bar-Or, A. et al. Induction of antigen-specific tolerance in multiple sclerosis after immunization with DNA encoding myelin basic protein in a randomized, placebo-controlled phase 1/2 trial. Arch. Neurol. 64, 1407–1415 (2007).
Bielekova, B. et al. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J. Immunol. 172, 3893–3904 (2004).
Kennedy, M. K. et al. Regulation of the effector stages of experimental autoimmune encephalomyelitis via neuroantigen-specific tolerance induction. J. Immunol. 145, 117–126 (1990).
Kennedy, M. K. et al. Inhibition of murine relapsing experimental autoimmune encephalomyelitis by immune tolerance to proteolipid protein and its encephalitogenic peptides. J. Immunol. 144, 909–915 (1990).
Tan, L. J. et al. Successful treatment of paralytic relapses in adoptive experimental autoimmune encephalomyelitis via neuroantigen-specific tolerance. J. Immunol. 147, 1797–1802 (1991).
Tan, L. J., Kennedy, M. K. & Miller, S. D. Regulation of the effector stages of experimental autoimmune encephalomyelitis via neuroantigen-specific tolerance induction. II. Fine specificity of effector T cell inhibition. J. Immunol. 148, 2748–2755 (1992).
Menges, M. et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 195, 15–21 (2002).
Trumpfheller, C. et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J. Exp. Med. 203, 607–617 (2006).
Soares, H. et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 204, 1095–1106 (2007).
Nchinda, G. et al. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J. Clin. Invest. 118, 1427–1436 (2008).
Jiang, W. et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 375, 151–155 (1995).
Bonifaz, L. et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196, 1627–1638 (2002).
Hawiger, D. et al. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo. Immunity 20, 695–705 (2004).
Burgdorf, S. et al. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316, 612–616 (2007).
Tacken, P. J. et al. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood 106, 1278–1285 (2005).
Meyer-Wentrup, F. et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-α production. Blood 111, 4245–4253 (2008).
Sancho, D. et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J. Clin. Invest. 118, 2098–2110 (2008).
Kaden, S. A. et al. Enhanced dendritic cell-induced immune responses mediated by the novel C-type lectin receptor mDCAR1. J. Immunol. 183, 5069–5078 (2009).
Dzionek, A. et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J. Exp. Med. 194, 1823–1834 (2001).
Mukhopadhaya, A. et al. Selective delivery of β cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc. Natl Acad. Sci. USA 105, 6374–6379 (2008).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–779 (2001).
Mahnke, K. et al. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood 101, 4862–4869 (2003).
Tarbell, K. V. et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J. Exp. Med. 204, 191–201 (2007).
Kappos, L. et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat. Med. 6, 1176–1182 (2000).
Bielekova, B. et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 6, 1167–1175 (2000).
Piemonti, L. et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J. Immunol. 164, 4443–4451 (2000).
Griffin. M. D. Dendritic cell modulation by 1α, 25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc. Natl Acad. Sci. USA 98, 6800–6805 (2001).
Benkhoucha, M. et al. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA 107, 6424–6429 (2010).
Rivas-Caicedo, A. et al. Jak3 is involved in dendritic cell maturation and CCR7-dependent migration. PLoS ONE 4, e7066 (2009).
Walker, J. G. et al. Characterisation of a dendritic cell subset in synovial tissue which strongly expresses Jak/STAT transcription factors from patients with rheumatoid arthritis. Ann. Rheum. Dis. 66, 992–999 (2007).
Boy, M. G. et al. Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690, 550 in patients with psoriasis. J. Invest. Dermatol. 129, 2299–2302 (2009).
Kremer, J. M. et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690, 550 versus placebo. Arthritis Rheum. 60, 1895–1905 (2009).
Steinman, R. M. & Idoyaga, J. Features of the dendritic cell lineage. Immunol. Rev. 234, 5–17 (2010).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Münz, C., Steinman, R. M. & Fujii, S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J. Exp. Med. 202, 203–207 (2005).
Gilliet, M., Cao, W. & Liu, Y. J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 8, 594–606 (2008).
Serbina, N. V., Jia, T., Hohl, T. M. & Pamer, E. G. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol. 26, 421–452 (2008).
Heath, W. R. & Carbone, F. R. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat. Immunol. 10, 1237–1244 (2009).
Robbins, S. H. et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 9, R17 (2008).
Acknowledgements
We thank B. Becher (Institute of Experimental Immunology, University of Zürich, Switzerland) for critically reviewing this manuscript. C. Münz is funded by the National Cancer Institute, the Foundation for the National Institutes of Health (Grand Challenges in Global Health) and the Swiss National Research Foundation. J. D. Lünemann is funded by the Swiss National Research Foundation, the Gemeinnützige Hertie Stiftung, the Swiss Multiple Sclerosis Foundation, the Betty and David Koetser Foundation, the Ernst Schering Foundation and the Baxter Research Grant Program.
Author information
Authors and Affiliations
Contributions
M. Comabella, X. Montalban, C. Münz and J. D. Lünemann all researched the data for the article, provided substantial contributions to discussions of the content, and contributed equally to the reviewing and/or editing of the manuscript before submission of the article. M. Comabella, C. Münz and J. D. Lünemann also contributed equally to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
M. Comabella has received has received honoraria from Bayer Schering Pharma, Biogen Idec, Merck Serono, Novartis, Sanofi-Aventis, Teva for lectures. X. Montalban has received honoraria from Almirall, Bayer Schering Pharma, Biogen Idec, Genentech, Genzyme, Merck Serono, Novartis, Sanofi-Aventis, Teva for lectures and consulting. He has also received research funding from Almirall, Bayer Schering Pharma, Biogen Idec, Genentech, Genzyme, Merck Serono, Novartis, Sanofi-Aventis, Teva. J. D. Lünemann has received honoraria from Baxter, Bayer Schering Pharma, Merck Serano, Talecris for lectures. He has also received research funding from Baxter and Bayer Schering Pharma. C. Münz declares no competing interests.
Rights and permissions
About this article
Cite this article
Comabella, M., Montalban, X., Münz, C. et al. Targeting dendritic cells to treat multiple sclerosis. Nat Rev Neurol 6, 499–507 (2010). https://doi.org/10.1038/nrneurol.2010.112
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneurol.2010.112
This article is cited by
-
CXCR1 drives the pathogenesis of EAE and ARDS via boosting dendritic cells-dependent inflammation
Cell Death & Disease (2023)
-
Impact of disease-modifying therapy on dendritic cells and exploring their immunotherapeutic potential in multiple sclerosis
Journal of Neuroinflammation (2022)
-
Dendritic Cell–Targeted Therapies to Treat Neurological Disorders
Molecular Neurobiology (2022)
-
Amlexanox attenuates experimental autoimmune encephalomyelitis by inhibiting dendritic cell maturation and reprogramming effector and regulatory T cell responses
Journal of Neuroinflammation (2019)
-
Discovery of BVDU as a promising Drug for autoimmune diseases Therapy by Dendritic-cell-based functional screening
Scientific Reports (2017)