Key Points
-
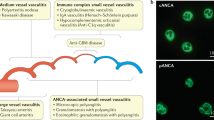
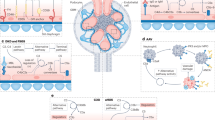
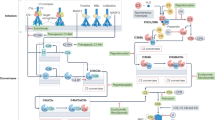
Activation of the complement system through the alternative pathway is necessary for the development of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) in animal models
-
C5a has a key role in AAV, linking inflammation and the coagulation system
-
Neutrophils, ANCA and the complement system form a positive feedback loop in the development of AAV
-
Inhibition of the complement system, particularly by targeting C5a, is a potential therapeutic approach for AAV
Abstract
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of potentially life-threatening autoimmune diseases. The main histological feature in the kidneys of patients with AAV is pauci-immune necrotizing crescentic glomerulonephritis with little immunoglobulin and complement deposition in the glomerular capillary walls. The complement system was not, therefore, initially thought to be associated with the development of AAV. Accumulating evidence from animal models and clinical observations indicate, however, that activation of the complement system — and the alternative pathway in particular — is crucial for the development of AAV, and that the complement activation product C5a has a central role. Stimulation of neutrophils with C5a and ANCA not only results in the neutrophil respiratory burst and degranulation, but also activates the coagulation system and generates thrombin, thus bridging the inflammation and coagulation systems. In this Review, we provide an overview of the clinical, in vivo and in vitro evidence for a role of complement activation in the development of AAV and discuss how targeting the complement system could provide opportunities for therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jennette, J. C. et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 65, 1–11 (2013).
Hagen, E. C. et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis. EC/BCR Project for ANCA Assay Standardization. Kidney Int. 53, 743–753 (1998).
Walport, M. J. Complement. First of two parts. N. Engl. J. Med. 344, 1058–1066 (2001).
Walport, M. J. Complement. Second of two parts. N. Engl. J. Med. 344, 1140–1144 (2001).
Amara, U. et al. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 185, 5628–5636 (2010).
Conway, E. M. Reincarnation of ancient links between coagulation and complement. J. Thromb. Haemost. 13 (Suppl. 1), S121–S132 (2015).
Nataf, S., Davoust, N., Ames, R. S. & Barnum, S. R. Human T cells express the C5a receptor and are chemoattracted to C5a. J. Immunol. 1950, 4018–4023 (1999).
Ricklin, D., Reis, E. S. & Lambris, J. D. Complement in disease: a defence system turning offensive. Nat. Rev. Nephrol. 12, 383–401 (2016). Comprehensive review that summarizes the complement system, including key activating mechanisms, crosstalk, the involvement of complement in clinical conditions and promising therapeutic approaches.
Xiao, H. et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J. Clin. Invest. 110, 955–963 (2002).
Schreiber, A., Xiao, H., Falk, R. J. & Jennette, J. C. Bone marrow-derived cells are sufficient and necessary targets to mediate glomerulonephritis and vasculitis induced by anti-myeloperoxidase antibodies. J. Am. Soc. Nephrol. 17, 3355–3364 (2006).
Little, M. A. et al. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte–microvascular interactions in vivo. Blood 106, 2050–2058 (2005).
Kettritz, R. How anti-neutrophil cytoplasmic autoantibodies activate neutrophils. Clin. Exp. Immunol. 169, 220–228 (2012).
Jayne, D. R. et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J. Am. Soc. Nephrol. 18, 2180–2188 (2007).
de Lind van Wijngaarden, R. A. et al. Chances of renal recovery for dialysis-dependent ANCA-associated glomerulonephritis. J. Am. Soc. Nephrol. 18, 2189–2197 (2007).
Xiao, H. et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am. J. Pathol. 167, 39–45 (2005).
Kessenbrock, K. et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 15, 623–625 (2009).
Lande, R. et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569 (2007).
Sangaletti, S. et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood 120, 3007–3018 (2012).
Xiao, H., Schreiber, A., Heeringa, P., Falk, R. J. & Jennette, J. C. Alternative complement pathway in the pathogenesis of disease mediated by antineutrophil cytoplasmic autoantibodies. Am. J. Pathol. 170, 52–64 (2007). This is the first study that highlighted the crucial role of the complement system in the pathogenesis of ANCA-associated vasculitis.
Huugen, D. et al. Inhibition of complement factor C5 protects against antimyeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 71, 646–654 (2007).
Schreiber, A. et al. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 20, 289–298 (2009).
Xiao, H. et al. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J. Am. Soc. Nephrol. 25, 225–231 (2014). This paper identified the role of the central complement component, C5a, in the pathogenesis of ANCA-associated vasculitis.
Haas, M. & Eustace, J. A. Immune complex deposits in ANCA-associated crescentic glomerulonephritis: a study of 126 cases. Kidney Int. 65, 2145–2152 (2004).
Yu, F. et al. Clinical and pathological features of renal involvement in propylthiouracil-associated ANCA-positive vasculitis. Am. J. Kidney Dis. 49, 607–614 (2007).
Chen, M., Xing, G. Q., Yu, F., Liu, G. & Zhao, M. H. Complement deposition in renal histopathology of patients with ANCA-associated pauci-immune glomerulonephritis. Nephrol. Dial. Transplant. 24, 1247–1252 (2009).
Xing, G. Q. et al. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J. Clin. Immunol. 29, 282–291 (2009).
Gou, S. J., Yuan, J., Wang, C., Zhao, M. H. & Chen, M. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin. J. Am. Soc. Nephrol. 8, 1884–1891 (2013).
Hilhorst, M. et al. Complement in ANCA-associated glomerulonephritis. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfv288 (2015).
Yuan, J. et al. C5a and its receptors in human anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Arthritis Res. Ther. 14, R140 (2012).
Huey, R. & Hugli, T. E. Characterization of a C5a receptor on human polymorphonuclear leukocytes (PMN). J. Immunol. 135, 2063–2068 (1985).
Gou, S. J., Yuan, J., Chen, M., Yu, F. & Zhao, M. H. Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int. 83, 129–137 (2013).
Manenti, L. et al. Association of serum C3 concentration and histologic signs of thrombotic microangiopathy with outcomes among patients with ANCA-associated renal vasculitis. Clin. J. Am. Soc. Nephrol. 10, 2143–2151 (2015).
Chen, S. F. et al. Clinicopathologic characteristics and outcomes of renal thrombotic microangiopathy in anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis. Clin. J. Am. Soc. Nephrol. 10, 750–758 (2015).
Noris, M. & Remuzzi, G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 361, 1676–1687 (2009).
Ferreira, V. P. et al. The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J. Immunol. 182, 7009–7018 (2009).
Servais, A. et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 82, 454–464 (2012).
Chen, S. F. et al. Plasma complement factor H is associated with disease activity of patients with ANCA-associated vasculitis. Arthritis Res. Ther. 17, 129 (2015).
Hao, J., Meng, L. Q., Xu, P. C., Chen, M. & Zhao, M. H. p38MAPK, ERK and PI3K signaling pathways are involved in C5a-primed neutrophils for ANCA-mediated activation. PLoS ONE 7, e38317 (2012).
Hao, J., Chen, M. & Zhao, M. H. Involvement of protein kinase C in C5a-primed neutrophils for ANCA-mediated activation. Mol. Immunol. 54, 68–73 (2013).
Kalant, D. et al. C5L2 is a functional receptor for acylation-stimulating protein. J. Biol. Chem. 280, 23936–23944 (2005).
Gao, H. et al. Evidence for a functional role of the second C5a receptor C5L2. FASEB J. 19, 1003–1005 (2005).
Chen, N. J. et al. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature 446, 203–207 (2007).
Rittirsch, D. et al. Functional roles for C5a receptors in sepsis. Nat. Med. 14, 551–557 (2008).
Hao, J., Wang, C., Yuan, J., Chen, M. & Zhao, M. H. A pro-inflammatory role of C5L2 in C5a-primed neutrophils for ANCA-induced activation. PLoS ONE 8, e66305 (2013).
Li, R., Coulthard, L. G., Wu, M. C., Taylor, S. M. & Woodruff, T. M. C5L2: a controversial receptor of complement anaphylatoxin, C5a. FASEB J. 27, 855–864 (2013).
Niessen, F. et al. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature 452, 654–658 (2008).
Choi, J. W. et al. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc. Natl Acad. Sci. USA 108, 751–756 (2011).
Samy, E. T. et al. Cutting edge: modulation of intestinal autoimmunity and IL-2 signaling by sphingosine kinase 2 independent of sphingosine 1-phosphate. J. Immunol. 179, 5644–5648 (2007).
Bachmaier, K., Guzman, E., Kawamura, T., Gao, X. & Malik, A. B. Sphingosine kinase 1 mediation of expression of the anaphylatoxin receptor C5L2 dampens the inflammatory response to endotoxin. PLoS ONE 7, e30742 (2012).
Hao, J., Huang, Y. M., Zhao, M. H. & Chen, M. The interaction between C5a and sphingosine-1-phosphate in neutrophils for antineutrophil cytoplasmic antibody mediated activation. Arthritis Res. Ther. 16, R142 (2014).
Seong, S. Y. & Matzinger, P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 4, 469–478 (2004).
Wang, C. et al. Association of circulating level of high mobility group box 1 with disease activity in antineutrophil cytoplasmic autoantibody–associated vasculitis. Arthritis Care Res. 65, 1828–1834 (2013).
Ma, T. T. et al. Urinary levels of high mobility group box-1 are associated with disease activity in antineutrophil cytoplasmic autoantibody-associated vasculitis. PLoS ONE 10, e0123586 (2015).
Wang, C. et al. High mobility group box 1 contributes to anti-neutrophil cytoplasmic antibody-induced neutrophils activation through receptor for advanced glycation end products (RAGE) and Toll-like receptor 4. Arthritis Res. Ther. 17, 64 (2015).
Ma, Y. H. et al. High-mobility group box 1 potentiates antineutrophil cytoplasmic antibody-inducing neutrophil extracellular traps formation. Arthritis Res. Ther. 18, 2 (2016).
Wang, C. et al. Involvement of high mobility group box 1 in the activation of C5a-primed neutrophils induced by ANCA. Clin. Immunol. 159, 47–57 (2015).
Stassen, P. M., Derks, R. P., Kallenberg, C. G. & Stegeman, C. A. Venous thromboembolism in ANCA-associated vasculitis — incidence and risk factors. Rheumatology (Oxford) 47, 530–534 (2008).
Merkel, P. A. et al. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener's Clinical Occurrence of Thrombosis (WeCLOT) Study. Ann. Intern. Med. 142, 620–626 (2005).
Ma, T. T., Huang, Y. M., Wang, C., Zhao, M. H. & Chen, M. Coagulation and fibrinolysis index profile in patients with ANCA-associated vasculitis. PLoS ONE 9, e97843 (2014).
Huang, Y. M., Wang, H., Wang, C., Chen, M. & Zhao, M. H. Promotion of hypercoagulability in antineutrophil cytoplasmic antibody-associated vasculitis by C5a-induced tissue factor-expressing microparticles and neutrophil extracellular traps. Arthritis Rheumatol. 67, 2780–2790 (2015). This paper found that C5a links the inflammation and coagulation process in AAV.
Camous, L. et al. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood 117, 1340–1349 (2011).
Leffler, J. et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 188, 3522–3531 (2012).
Wang, H., Wang, C., Zhao, M. H. & Chen, M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 181, 518–527 (2015).
Pfister, H. et al. Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood 104, 1411–1418 (2004).
van der Geld, Y. M. et al. Rats and mice immunised with chimeric human/mouse proteinase 3 produce autoantibodies to mouse Pr3 and rat granulocytes. Ann. Rheum. Dis. 66, 1679–1682 (2007).
Kambas, K. et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann. Rheum. Dis. 73, 1854–1863 (2014).
Ritis, K. et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 177, 4794–4802 (2006).
Huber-Lang, M. et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat. Med. 12, 682–687 (2006).
Ekdahl, K. N. et al. Dangerous liaisons: complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol. Rev. 274, 245–269 (2016).
Speth, C. et al. Complement and platelets: mutual interference in the immune network. Mol. Immunol. 67, 108–118 (2015).
Lood, C. et al. Platelet activation and anti-phospholipid antibodies collaborate in the activation of the complement system on platelets in systemic lupus erythematosus. PLoS ONE 9, e99386 (2014).
Martel, C. et al. Requirements for membrane attack complex formation and anaphylatoxins binding to collagen-activated platelets. PLoS ONE 6, e18812 (2011).
Sims, P. J. & Wiedmer, T. Induction of cellular procoagulant activity by themem-brane attack complex of complement. Semin. Cell Biol. 6, 275–282 (1995).
Del Conde, I., Crúz, M. A., Zhang, H., López, J. A. & Afshar-Kharghan, V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 201, 871–879 (2005).
Willeke, P. et al. Platelet counts as a biomarker in ANCA-associated vasculitis. Scand. J. Rheumatol. 44, 302–308 (2015).
Mogensen, T. H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22, 240–273 (2009).
Iwasaki, A. & Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 16, 343–353 (2015). This comprehensive review discusses the emerging principles of innate control of adaptive immunity.
Raby, A. C. et al. TLR activation enhances C5a-induced pro-inflammatory responses by negatively modulating the second C5a receptor, C5L2. Eur. J. Immunol. 41, 2741–2752 (2011).
Zou, L. et al. Complement factor B is the downstream effector of TLRs and plays an important role in a mouse model of severe sepsis. J. Immunol. 191, 5625–5635 (2013).
Huugen, D. et al. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-alpha. Am. J. Pathol. 167, 47–58 (2005).
Wang, H., Gou, S. J., Zhao, M. H. & Chen, M. The expression of Toll-like receptors 2, 4 and 9 in kidneys of patients with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Clin. Exp. Immunol. 177, 603–610 (2014).
Wang, C. et al. Emerging role of high mobility group box 1 in ANCA-associated vasculitis. Autoimmun. Rev. 14, 1057–1065 (2015).
Woodruff, T. M., Nandakumar, K. S. & Tedesco, F. Inhibiting the C5-C5a receptor axis. Mol. Immunol. 48, 1631–1642 (2011).
Hillmen, P. et al. Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 350, 552–559 (2004).
Hillmen, P. et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 355, 1233–1243 (2006).
Legendre, C. M. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013).
Vivarelli, M. & Emma, F. Treatment of C3 glomerulopathy with complement blockers. Semin. Thromb. Hemost. 40, 472–477 (2014).
Rosenblad, T. et al. Eculizumab treatment for rescue of renal function in IgA nephropathy. Pediatr. Nephrol. 29, 2225–2228 (2014).
Pickering, M. C. et al. Eculizumab as rescue therapy in severe resistant lupus nephritis. Rheumatology (Oxford) 54, 2286–2288 (2015).
Shapira, I., Andrade, D., Allen, S. L. & Salmon, J. E. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 64, 2719–2723 (2012).
Castellano, G. et al. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. Am. J. Pathol. 176, 1648–1659 (2010).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01147302 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01134510 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02134314 (2017).
Paixão-Cavalcante, D. et al. A humanized antibody that regulates the alternative pathway convertase: potential for therapy of renal disease associated with nephritic factors. J. Immunol. 192, 4844–4851 (2014).
Zhang, Y. et al. Soluble CR1 therapy improves complement regulation in C3 glomerulopathy. J. Am. Soc. Nephrol. 24, 1820–1829 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01363388 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02222155 (2016).
Acknowledgements
The authors' work is supported by three grants of the National Natural Science Fund (No. 81425008, No. 81621092 and No. 81370829) and a grant from the National Key Research and Development Program (No. 2016YFC0906102). We are very grateful to Dr. Chen Wang for drawing the first draft of figures for this article. David Jayne is supported by the Cambridge Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided substantial contributions to discussions of the content, wrote the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Polyangiitis
-
Inflammation involving multiple blood vessels.
- Vasculitides
-
A group of disorders that destroy blood vessels by inflammation. Both arteries and veins are affected.
- Fluid phase
-
In the context of complement activation, fluid phase refers to activation in the circulation, rather than on the surface of cells.
- Properdin
-
The only known positive regulator of complement activation that stabilizes the alternative pathway convertases.
- Glomerular capillary tuft infarction
-
Occlusion of the lumen of glomerular capillaries by micro-thrombi.
- Opsonize
-
To make bacteria or other cells more susceptible to the action of phagocytes via a mechanism involving binding of an opsonin, such as IgG or C3b
- Plasma exchange
-
Removal, treatment and return or exchange of blood plasma or components from and to the circulation; often used to treat severe autoimmune diseases.
Rights and permissions
About this article
Cite this article
Chen, M., Jayne, D. & Zhao, MH. Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat Rev Nephrol 13, 359–367 (2017). https://doi.org/10.1038/nrneph.2017.37
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2017.37
This article is cited by
-
Lymphatic vessels in patients with crescentic glomerulonephritis: association with renal pathology and prognosis
Journal of Nephrology (2024)
-
Die Komplementkaskade in der Nierenpathologie
Die Pathologie (2024)
-
Low platelet count at diagnosis of anti-neutrophil cytoplasmic antibody-associated vasculitis is correlated with the severity of disease and renal prognosis
Clinical and Experimental Medicine (2024)
-
Coexistence of cryoglobulinemia and ANCA-associated vasculitis in a chronic brucellosis patient -a case report and literature review
BMC Infectious Diseases (2023)
-
Mechanism of activation and biased signaling in complement receptor C5aR1
Cell Research (2023)