Key Points
-
The introduction of biologics that are safer and less toxic than conventional immunosuppressive therapies has markedly improved patient outcomes for many common autoimmune diseases
-
Good evidence suggests that many biologics will be effective in autoimmune renal diseases, but their development and introduction into clinical practice has been remarkably slow
-
Clinical and basic research is required to define the dominant disease-promoting molecules that underlie autoimmune renal diseases and could be targeted by current and evolving biologics
-
An increase in the size of patient cohorts and the assembly of clinician networks will facilitate the design and execution of multicentre, multinational, randomized controlled trials for new biologics
-
A strategic approach must be adopted to define the appropriate patient subgroups that will help prioritize which biologics should be tested, based on the available evidence of likely efficacy
Abstract
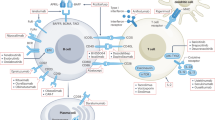
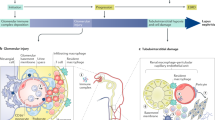
Biological therapeutics (biologics) that target autoimmune responses and inflammatory injury pathways have a marked beneficial impact on the management of many chronic diseases, including rheumatoid arthritis, psoriasis, inflammatory bowel disease, and ankylosing spondylitis. Accumulating data suggest that a growing number of renal diseases result from autoimmune injury — including lupus nephritis, IgA nephropathy, anti-neutrophil cytoplasmic antibody-associated glomerulonephritis, autoimmune (formerly idiopathic) membranous nephropathy, anti-glomerular basement membrane glomerulonephritis, and C3 nephropathy — and one can speculate that biologics might also be applicable to these diseases. As many autoimmune renal diseases are relatively uncommon, with long natural histories and diverse outcomes, clinical trials that aim to validate potentially useful biologics are difficult to design and/or perform. Some excellent consortia are undertaking cohort studies and clinical trials, but more multicentre international collaborations are needed to advance the introduction of new biologics to patients with autoimmune renal disorders. This Review discusses the key molecules that direct injurious inflammation and the biologics that are available to modulate them. The opportunities and challenges for the introduction of relevant biologics into treatment protocols for autoimmune renal diseases are also discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Furst, D. E. et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2012. Ann. Rheum. Dis. 72 (Suppl. 2), ii2–ii34 (2013).
Couser, W. G. Basic and translational concepts of immune-mediated glomerular diseases. J. Am. Soc. Nephrol. 23, 381–399 (2012).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 (2011).
Couser, W. G. & Johnson, R. J. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int. 86, 905–914 (2014).
Yeo, S. C. & Liew, A. Biologic agents in the treatment of glomerulonephritides. Nephrology (Carlton) 20, 767–787 (2015).
Naik, A. et al. Complement regulation in renal disease models. Semin.. Nephrol. 33, 575–585 (2013).
Emancipator, S. N. Animal models of IgA nephropathy. Curr. Protoc. Immunol. 15, 15.11 (2001).
Du, Y., Sanam, S., Kate, K. & Mohan, C. Animal models of lupus and lupus nephritis. Curr. Pharm. Des. 21, 2320–2349 (2015).
Borza, D. B. et al. Mouse models of membranous nephropathy: the road less travelled by. Am. J. Clin. Exp. Immunol. 2, 135–145 (2013).
Ooi, J. D., Gan, P. Y., Odobasic, D., Holdsworth, S. R. & Kitching, A. R. T cell mediated autoimmune glomerular disease in mice. Curr. Protoc. Immunol. 107, 15.27.1–15.27.19 (2014).
Odobasic, D., Ghali, J. R., O'Sullivan, K. M., Holdsworth, S. R. & Kitching, A. R. Glomerulonephritis induced by heterologous anti-GBM globulin as a planted foreign antigen. Curr. Protoc. Immunol. 106, 15.26.1–15.26.20 (2014).
Gutcher, I. & Becher, B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Invest. 117, 1119–1127 (2007).
Holdsworth, S. R., Kitching, A. R. & Tipping, P. G. Th1 and Th2 T helper cell subsets affect patterns of injury and outcomes in glomerulonephritis. Kidney Int. 55, 1198–1216 (1999).
Kitching, A. R. & Holdsworth, S. R. The emergence of Th17 cells as effectors of renal injury. J. Am. Soc. Nephrol. 22, 235–238 (2011).
Browning, J. L. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat. Rev. Drug Discov. 5, 564–576 (2006).
Dorner, T. et al. Current status on B-cell depletion therapy in autoimmune diseases other than rheumatoid arthritis. Autoimmun. Rev. 9, 82–89 (2009).
Iwata, Y. et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 117, 530–541 (2011).
Janeway, C. A., Travers, P., Walport, M. & Schlomchick, M. J. Immunobiology: The immune System in Health and Disease 5th edn (Garland Science, 2001).
Tipping, P. G. & Holdsworth, S. R. Cytokines in glomerulonephritis. Semin. Nephrol. 27, 275–285 (2007).
Polman, C. H. et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899–910 (2006).
Rudick, R. A. et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N. Engl. J. Med. 354, 911–923 (2006).
Deshmane, S. L., Kremlev, S., Amini, S. & Sawaya, B. E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interferon Cytokine Res. 29, 313–326 (2009).
Kalinowska, A. & Losy, J. Investigational C-C chemokine receptor 2 antagonists for the treatment of autoimmune diseases. Expert Opin. Investig. Drugs 17, 1267–1279 (2008).
Brodmerkel, C. M. et al. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J. Immunol. 175, 5370–5378 (2005).
Kulkarni, O. et al. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J. Am. Soc. Nephrol. 18, 2350–2358 (2007).
Gong, J. H., Ratkay, L. G., Waterfield, J. D. & Clark-Lewis, I. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J. Exp. Med. 186, 131–137 (1997).
Legendre, C. M. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013).
Rathbone, J. et al. A systematic review of eculizumab for atypical haemolytic uraemic syndrome (aHUS). BMJ Open 3, e003573 (2013).
Tillmanns, S. et al. SM101, a novel recombinant, soluble, human FcγIIB receptor, in the treatment of systemic lupus erythematosus: results of a double-blline, placebo-controlled multicenter study. Am. Coll. Rheumatol. 66, S1238 (2014).
van de Wiel, B. A. et al. Interference of Wegener's granulomatosis autoantibodies with neutrophil proteinase 3 activity. Clin. Exp. Immunol. 90, 409–414 (1992).
Jennette, J. C. & Falk, R. J. Small-vessel vasculitis. N. Engl. J. Med. 337, 1512–1523 (1997).
Lyons, P. A. et al. Genetically distinct subsets within ANCA-associated vasculitis. N. Engl. J. Med. 367, 214–223 (2012).
Xiao, H. et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J. Clin. Invest. 110, 955–963 (2002).
Huugen, D. et al. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-α. Am. J. Pathol. 167, 47–58 (2005).
Stone, J. H. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N. Engl. J. Med. 363, 221–232 (2010).
Jones, R. B. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N. Engl. J. Med. 363, 211–220 (2010).
Guillevin, L. et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N. Engl. J. Med. 371, 1771–1780 (2014).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Oflazoglu, E. & Audoly, L. P. Evolution of anti-CD20 monoclonal antibody therapeutics in oncology. MAbs 2, 14–19 (2010).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Schneeweis, C. et al. Increased levels of BLyS and sVCAM-1 in anti-neutrophil cytoplasmatic antibody (ANCA)-associated vasculitides (AAV). Clin. Exp. Rheumatol. 28, 62–66 (2010).
Krumbholz, M. et al. BAFF is elevated in serum of patients with Wegener's granulomatosis. J. Autoimmun. 25, 298–302 (2005).
Bader, L., Koldingsnes, W. & Nossent, J. B-lymphocyte activating factor levels are increased in patients with Wegener's granulomatosis and inversely correlated with ANCA titer. Clin. Rheumatol. 29, 1031–1035 (2010).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Stilmant, M. M., Bolton, W. K., Sturgill, B. C., Schmitt, G. W. & Couser, W. G. Crescentic glomerulonephritis without immune deposits: clinicopathologic features. Kidney Int. 15, 184–195 (1979).
Huugen, D. et al. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 71, 646–654 (2007).
Xiao, H. et al. C5a receptor (CD88) blockade protects against MPO–ANCA GN. J. Am. Soc. Nephrol. 25, 225–231 (2014).
Schreiber, A. et al. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J. Am. Soc. Nephrol. 20, 289–298 (2009).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Romo-Tena, J., Gomez-Martin, D. & Alcocer-Varela, J. CTLA-4 and autoimmunity: new insights into the dual regulator of tolerance. Autoimmun. Rev. 12, 1171–1176 (2013).
Abrams, J. R. et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J. Exp. Med. 192, 681–694 (2000).
Langford, C. A. et al. An open-label trial of abatacept (CTLA4-IG) in non-severe relapsing granulomatosis with polyangiitis (Wegener's). Ann. Rheum. Dis. 73, 1376–1379 (2014).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Moreland, L. W. et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N. Engl. J. Med. 337, 141–147 (1997).
Moelants, E. A., Mortier, A., Van Damme, J. & Proost, P. Regulation of TNF-α with a focus on rheumatoid arthritis. Immunol. Cell Biol. 91, 393–401 (2013).
Little, M. A. et al. Therapeutic effect of anti-TNF-α antibodies in an experimental model of anti-neutrophil cytoplasm antibody-associated systemic vasculitis. J. Am. Soc. Nephrol. 17, 160–169 (2006).
Stone, J. H. et al. Etanercept combined with conventional treatment in Wegener's granulomatosis: a six-month open-label trial to evaluate safety. Arthritis Rheum. 44, 1149–1154 (2001).
The Wegener's Granulomatosis Etanercept Tiral (WGET) Research Group. Etanercept plus standard therapy for Wegener's granulomatosis. N. Engl. J. Med. 352, 351–361 (2005).
Stone, J. H. et al. Solid malignancies among patients in the Wegener's Granulomatosis Etanercept Trial. Arthritis Rheum. 54, 1608–1618 (2006).
Mukhtyar, C. & Luqmani, R. Current state of tumour necrosis factor α blockade in Wegener's granulomatosis. Ann. Rheum. Dis. 64, iv31–iv36 (2005).
Lamprecht, P. et al. Effectiveness of TNF-α blockade with infliximab in refractory Wegener's granulomatosis. Rheumatol. (Oxford) 41, 1303–1307 (2002).
Booth, A. D., Jefferson, H. J., Ayliffe, W., Andrews, P. A. & Jayne, D. R. Safety and efficacy of TNFα blockade in relapsing vasculitis. Ann. Rheum. Dis. 61, 559 (2002).
Bartolucci, P. et al. Efficacy of the anti-TNF-α antibody infliximab against refractory systemic vasculitides: an open pilot study on 10 patients. Rheumatol. (Oxford) 41, 1126–1132 (2002).
Booth, A. et al. Prospective study of TNFα blockade with infliximab in anti-neutrophil cytoplasmic antibody-associated systemic vasculitis. J. Am. Soc. Nephrol. 15, 717–721 (2004).
US National Libary of Science. ClinicalTrials.gov[online], (2008).
Morgan, M. D., Drayson, M. T., Savage, C. O. & Harper, L. Addition of infliximab to standard therapy for ANCA-associated vasculitis. Nephron Clin. Pract. 117, c89–c97 (2011).
Smolen, J. S. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371, 987–997 (2008).
Arimura, Y. et al. Serum myeloperoxidase and serum cytokines in anti-myeloperoxidase antibody-associated glomerulonephritis. Clin. Nephrol. 40, 256–264 (1993).
Ohlsson, S., Wieslander, J. & Segelmark, M. Circulating cytokine profile in anti-neutrophilic cytoplasmatic autoantibody-associated vasculitis: prediction of outcome? Mediators Inflamm. 13, 275–283 (2004).
Berti, A. et al. Interleukin-6 in ANCA-associated vasculitis: rationale for successful treatment with tocilizumab. Semin. Arthritis Rheum. 45, 48–54 (2015).
Vaglio, A., Moosig, F. & Zwerina, J. Churg–Strauss syndrome: update on pathophysiology and treatment. Curr. Opin. Rheumatol. 24, 24–30 (2012).
Kahn, J. E. et al. Sustained response to mepolizumab in refractory Churg–Strauss syndrome. J. Allergy Clin. Immunol. 125, 267–270 (2010).
Kim, S., Marigowda, G., Oren, E., Israel, E. & Wechsler, M. E. Mepolizumab as a steroid-sparing treatment option in patients with Churg–Strauss syndrome. J. Allergy Clin. Immunol. 125, 1336–1343 (2010).
Herrmann, K., Gross, W. L. & Moosig, F. Extended follow-up after stopping mepolizumab in relapsing/refractory Churg–Strauss syndrome. Clin. Exp. Rheumatol. 30, S62–S65 (2012).
US National Libary of Science. ClinicalTrials.gov[online], (2009).
US National Libary of Science. ClinicalTrials.gov[online], (2012).
Giavina-Bianchi, P., Giavina-Bianchi, M., Agondi, R. & Kalil, J. Omalizumab and Churg–Strauss syndrome. J. Allergy Clin. Immunol. 122, 217; author reply 217–218 (2008).
Iglesias, E. et al. Successful management of Churg–Strauss syndrome using omalizumab as adjuvant immunomodulatory therapy: first documented pediatric case. Pediatr. Pulmonol. 49, E78–E81 (2014).
Pabst, S., Tiyerili, V. & Grohe, C. Apparent response to anti-IgE therapy in two patients with refractory 'forme fruste' of Churg–Strauss syndrome. Thorax 63, 747–748 (2008).
Mellors, R. C., Ortega, L. G. & Holman, H. R. Role of gamma globulins in pathogenesis of renal lesions in systemic lupus erythematosus and chronic membranous glomerulonephritis, with an observation on the lupus erythematosus cell reaction. J. Exp. Med. 106, 191–202 (1957).
Beck, L. H. Jr. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361, 11–21 (2009).
Tomas, N. M. et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N. Engl. J. Med. 371, 2277–2287 (2014).
Beck, L. H. Jr & Salant, D. J. Membranous nephropathy: from models to man. J. Clin. Invest. 124, 2307–2314 (2014).
Beck, L. H. Jr. et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J. Am. Soc. Nephrol. 22, 1543–1550 (2011).
Howman, A. et al. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet 381, 744–751 (2013).
Jha, V. et al. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 18, 1899–1904 (2007).
Heymann, W., Hackel, D. B., Harwood, S., Wilson, S. G. & Hunter, J. L. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc. Soc. Exp. Biol. Med. 100, 660–664 (1959).
Baker, P. J. et al. Depletion of C6 prevents development of proteinuria in experimental membranous nephropathy in rats. Am. J. Pathol. 135, 185–194 (1989).
Cunningham, P. N. & Quigg, R. J. Contrasting roles of complement activation and its regulation in membranous nephropathy. J. Am. Soc. Nephrol. 16, 1214–1222 (2005).
Noris, M., Mele, C. & Remuzzi, G. Podocyte dysfunction in atypical haemolytic uraemic syndrome. Nat. Rev. Nephrol. 11, 245–252 (2015).
Neale, T. J. et al. Tumor necrosis factor-α is expressed by glomerular visceral epithelial cells in human membranous nephropathy. Am. J. Pathol. 146, 1444–1454 (1995).
Ruggenenti, P. et al. Rituximab for idiopathic membranous nephropathy: who can benefit? Clin. J. Am. Soc. Nephrol. 1, 738–748 (2006).
Fervenza, F. C. et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 73, 117–125 (2008).
Fervenza, F. C. et al. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin. J. Am. Soc. Nephrol. 5, 2188–2198 (2010).
Cravedi, P., Ruggenenti, P., Sghirlanzoni, M. C. & Remuzzi, G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2, 932–937 (2007).
Segarra, A. et al. Successful treatment of membranous glomerulonephritis with rituximab in calcineurin inhibitor-dependent patients. Clin. J. Am. Soc. Nephrol. 4, 1083–1088 (2009).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Fervenza, F. C. et al. A multicenter randomized controlled trial of rituximab versus cyclosporine in the treatment of idiopathic membranous nephropathy (MENTOR). Nephron 130, 159–168 (2015).
Sethi, S. & Fervenza, F. C. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin. Nephrol. 31, 341–348 (2011).
Fakhouri, F., Fremeaux-Bacchi, V., Noel, L. H., Cook, H. T. & Pickering, M. C. C3 glomerulopathy: a new classification. Nat. Rev. Nephrol. 6, 494–499 (2010).
Sethi, S. & Fervenza, F. C. Membranoproliferative glomerulonephritis — a new look at an old entity. N. Engl. J. Med. 366, 1119–1131 (2012).
Zipfel, P. F. et al. The role of complement in C3 glomerulopathy. Mol. Immunol. 67, 21–30 (2015).
Pickering, M. C. et al. C3 glomerulopathy: consensus report. Kidney Int. 84, 1079–1089 (2013).
Sethi, S. et al. Mayo clinic/renal pathology society consensus report on pathologic classification, diagnosis, and reporting of GN. J. Am. Soc. Nephrol. http://dx.doi.org/10.1681/ASN.2015060612 (2015).
Servais, A. et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 82, 454–464 (2012).
Pickering, M. C. et al. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc. Natl Acad. Sci. USA 103, 9649–9654 (2006).
Sethi, S. et al. C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 82, 465–473 (2012).
Bomback, A. S. Eculizumab in the treatment of membranoproliferative glomerulonephritis. Nephron Clin. Pract. 128, 270–276 (2014).
Bomback, A. S. et al. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin. J. Am. Soc. Nephrol. 7, 748–756 (2012).
Herlitz, L. C. et al. Pathology after eculizumab in dense deposit disease and C3 GN. J. Am. Soc. Nephrol. 23, 1229–1237 (2012).
Radhakrishnan, S. et al. Eculizumab and refractory membranoproliferative glomerulonephritis. N. Engl. J. Med. 366, 1165–1166 (2012).
Vivarelli, M., Pasini, A. & Emma, F. Eculizumab for the treatment of dense-deposit disease. N. Engl. J. Med. 366, 1163–1165 (2012).
Daina, E., Noris, M. & Remuzzi, G. Eculizumab in a patient with dense-deposit disease. N. Engl. J. Med. 366, 1161–1163 (2012).
McCaughan, J. A., O'Rourke, D. M. & Courtney, A. E. Recurrent dense deposit disease after renal transplantation: an emerging role for complementary therapies. Am. J. Transplant. 12, 1046–1051 (2012).
Gurkan, S. et al. Eculizumab and recurrent C3 glomerulonephritis. Pediatr. Nephrol. 28, 1975–1981 (2013).
Rousset-Rouviere, C. et al. Rituximab fails where eculizumab restores renal function in C3nef-related DDD. Pediatr. Nephrol. 29, 1107–1111 (2014).
Ozkaya, O. et al. Eculizumab therapy in a patient with dense-deposit disease associated with partial lipodystropy. Pediatr. Nephrol. 29, 1283–1287 (2014).
Kerns, E., Rozansky, D. & Troxell, M. L. Evolution of immunoglobulin deposition in C3-dominant membranoproliferative glomerulopathy. Pediatr. Nephrol. 28, 2227–2231 (2013).
Nester, C. M. & Smith, R. J. Treatment options for C3 glomerulopathy. Curr. Opin. Nephrol. Hypertens. 22, 231–237 (2013).
US National Libary of Science. ClinicalTrials.gov[online], (2014).
Melis, J. P. et al. Complement in therapy and disease: regulating the complement system with antibody-based therapeutics. Mol. Immunol. 67, 117–130 (2015).
Ricklin, D. & Lambris, J. D. Complement in immune and inflammatory disorders: therapeutic interventions. J. Immunol. 190, 3839–3847 (2013).
Ruseva, M. M. et al. Efficacy of targeted complement inhibition in experimental C3 glomerulopathy. J. Am. Soc. Nephrol. 27, 405–416 (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2014).
Zhang, Y. et al. Soluble CR1 therapy improves complement regulation in C3 glomerulopathy. J. Am. Soc. Nephrol. 24, 1820–1829 (2013).
Schmidt, C. Q. et al. Rational engineering of a minimized immune inhibitor with unique triple-targeting properties. J. Immunol. 190, 5712–5721 (2013).
Hebecker, M. et al. An engineered construct combining complement regulatory and surface-recognition domains represents a minimal-size functional factor H. J. Immunol. 191, 912–921 (2013).
Angioi, A. et al. Diagnosis of complement alternative pathway disorders. Kidney Int. 89, 278–288 (2016).
Barratt, J. & Feehally, J. Primary IgA nephropathy: new insights into pathogenesis. Semin. Nephrol. 31, 349–360 (2011).
Mestecky, J. et al. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgA nephropathy. Contrib. Nephrol. 104, 172–182 (1993).
Gharavi, A. G. et al. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J. Am. Soc. Nephrol. 19, 1008–1014 (2008).
Suzuki, H. et al. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J. Clin. Invest. 119, 1668–1677 (2009).
Moura, I. C. et al. Identification of the transferrin receptor as a novel immunoglobulin (Ig)a1 receptor and its enhanced expression on mesangial cells in IgA nephropathy. J. Exp. Med. 194, 417–425 (2001).
Dohi, K. et al. The prognostic significance of urinary interleukin 6 in IgA nephropathy. Clin. Nephrol. 35, 1–5 (1991).
Lee, T. W., Ahn, J. H., Park, J. K., Ihm, C. G. & Kim, M. J. Tumor necrosis factor α from peripheral blood mononuclear cells of IgA nephropathy and mesangial cell proliferation. Kor. J. Intern. Med. 9, 1–8 (1994).
Xin, G. et al. Serum BAFF is elevated in patients with IgA nephropathy and associated with clinical and histopathological features. J. Nephrol. 26, 683–690 (2013).
Lin, F. J. et al. Imbalance of regulatory T cells to Th17 cells in IgA nephropathy. Scand. J. Clin. Lab. Invest. 72, 221–229 (2012).
Ohsawa, I. et al. Extraglomerular C3 deposition and metabolic impacts in patients with IgA nephropathy. Nephrol. Dial. Transplant. 28, 1856–1864 (2013).
Suzuki, H. et al. Fluctuation of serum C3 levels reflects disease activity and metabolic background in patients with IgA nephropathy. J. Nephrol. 26, 708–715 (2013).
Sugiura, H. et al. Effect of single-dose rituximab on primary glomerular diseases. Nephron Clin. Pract. 117, c98–c105 (2011).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Lamm, M. E. et al. Microbial IgA protease removes IgA immune complexes from mouse glomeruli in vivo: potential therapy for IgA nephropathy. Am. J. Pathol. 172, 31–36 (2008).
Cairns, L. S. et al. The fine specificity and cytokine profile of T-helper cells responsive to the α3 chain of type IV collagen in Goodpasture's disease. J. Am. Soc. Nephrol. 14, 2801–2812 (2003).
Ooi, J. D. et al. The HLA-DRB1*15:01-restricted Goodpasture's T cell epitope induces GN. J. Am. Soc. Nephrol. 24, 419–431 (2013).
Pedchenko, V. et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N. Engl. J. Med. 363, 343–354 (2010).
Chen, J. L. et al. Association of epitope spreading of antiglomerular basement membrane antibodies and kidney injury. Clin. J. Am. Soc. Nephrol. 8, 51–58 (2013).
Phelps, R. G. & Rees, A. J. The HLA complex in Goodpasture's disease: a model for analyzing susceptibility to autoimmunity. Kidney Int. 56, 1638–1653 (1999).
Wu, J. et al. CD4+ T cells specific to a glomerular basement membrane antigen mediate glomerulonephritis. J. Clin. Invest. 109, 517–524 (2002).
Ooi, J. D., Phoon, R. K., Holdsworth, S. R. & Kitching, A. R. IL-23, not IL-12, directs autoimmunity to the Goodpasture antigen. J. Am. Soc. Nephrol. 20, 980–989 (2009).
Hunemorder, S. et al. TH1 and TH17 cells promote crescent formation in experimental autoimmune glomerulonephritis. J. Pathol. 237, 62–71 (2015).
Salama, A. D. et al. Regulation by CD25+ lymphocytes of autoantigen-specific T-cell responses in Goodpasture's (anti-GBM) disease. Kidney Int. 64, 1685–1694 (2003).
Kidney Disease Improving Global Outcomes. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2, (Suppl. 2) 233–239 (2012).
Henderson, L. et al. Treatment for lupus nephritis. Cochrane Database Syst. Rev. 12, CD002922 (2012).
Finck, B. K., Linsley, P. S. & Wofsy, D. Treatment of murine lupus with CTLA4Ig. Science 265, 1225–1227 (1994).
Reap, E. A., Sobel, E. S., Cohen, P. L. & Eisenberg, R. A. Conventional B cells, not B-1 cells, are responsible for producing autoantibodies in lpr mice. J. Exp. Med. 177, 69–78 (1993).
Mackay, F. et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190, 1697–1710 (1999).
Santiago-Raber, M. L. et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J. Exp. Med. 197, 777–788 (2003).
Kiberd, B. A. Interleukin-6 receptor blockage ameliorates murine lupus nephritis. J. Am. Soc. Nephrol. 4, 58–61 (1993).
Merrill, J. et al. Assessment of flares in lupus patients enrolled in a Phase II/III study of rituximab (EXPLORER). Lupus 20, 709–716 (2011).
Rovin, B. H. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 64, 1215–1226 (2012).
Lightstone, L. The landscape after LUNAR: rituximab's crater-filled path. Arthritis Rheum. 64, 962–965 (2012).
Rovin, B. H. Targeting B-cells in lupus nephritis: should cautious optimism remain? Nephrol. Dial. Transplant. 28, 7–9 (2013).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Mysler, E. F. et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, Phase III study. Arthritis Rheum. 65, 2368–2379 (2013).
Gregersen, J. W. & Jayne, D. R. B-cell depletion in the treatment of lupus nephritis. Nat. Rev. Nephrol. 8, 505–514 (2012).
Al Rayes, H. & Touma, Z. Profile of epratuzumab and its potential in the treatment of systemic lupus erythematosus. Drug Des. Devel. Ther. 8, 2303–2310 (2014).
Navarra, S. V. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, Phase 3 trial. Lancet 377, 721–731 (2011).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Vincent, F. B. et al. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 24, 203–215 (2013).
US National Libary of Science. ClinicalTrials.gov[online], (2014).
Ginzler, E. M. et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res. Ther. 14, R33 (2012).
Furie, R. et al. Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol. 66, 379–389 (2014).
Askanase, A. D. et al. Treatment of lupus nephritis with abatacept: the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study. Arthritis Rheumatol. 66, 3096–3104 (2014).
Mohan, C., Shi, Y., Laman, J. D. & Datta, S. K. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J. Immunol. 154, 1470–1480 (1995).
Ruth, A. J. et al. An IL-12-independent role for CD40–CD154 in mediating effector responses: studies in cell-mediated glomerulonephritis and dermal delayed-type hypersensitivity. J. Immunol. 173, 136–144 (2004).
Ruth, A. J., Kitching, A. R., Semple, T. J., Tipping, P. G. & Holdsworth, S. R. Intrinsic renal cell expression of CD40 directs Th1 effectors inducing experimental crescentic glomerulonephritis. J. Am. Soc. Nephrol. 14, 2813–2822 (2003).
Sidiropoulos, P. I. & Boumpas, D. T. Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus 13, 391–397 (2004).
Jacob, C. O. & McDevitt, H. O. Tumour necrosis factor-α in murine autoimmune 'lupus' nephritis. Nature 331, 356–358 (1988).
Aringer, M. & Smolen, J. S. Therapeutic blockade of TNF in patients with SLE — promising or crazy? Autoimmun. Rev. 11, 321–325 (2012).
US National Libary of Science. ClinicalTrials.gov[online], (2013).
US National Libary of Science. ClinicalTrials.gov[online], (2009).
Michaelson, J. S., Wisniacki, N., Burkly, L. C. & Putterman, C. Role of TWEAK in lupus nephritis: a bench-to-bedside review. J. Autoimmun. 39, 130–142 (2012).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Bennett, L. et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 197, 711–723 (2003).
Baechler, E. C. et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl Acad. Sci. USA 100, 2610–2615 (2003).
Zheng, B., Yu, X. Q., Greth, W. & Robbie, G. J. Population pharmacokinetic analysis of sifalimumab from a clinical Phase IIb trial in systemic lupus erythematosus patients. Br. J. Clin. Pharmacol. (2015).
Kalunian, K. C. et al. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 75, 196–202 (2016).
Fukatsu, A. et al. Distribution of interleukin-6 in normal and diseased human kidney. Lab. Invest. 65, 61–66 (1991).
Ryffel, B. et al. Interleukin-6 exacerbates glomerulonephritis in (NZB x NZW)F1 mice. Am. J. Pathol. 144, 927–937 (1994).
US National Libary of Science. ClinicalTrials.gov[online], (2014).
van Vollenhoven, R. et al. A Phase 2, multicenter, randomized, double-blind, placebo-controlled, proof-of-concept study to evaluate the efficacy and safety of sirukumab in patients with active lupus nephritis. Ann. Rheum. Dis. 73 (Suppl. 2), 78 (2014).
Illei, G. G. et al. Tocilizumab in systemic lupus erythematosus: data on safety, preliminary efficacy, and impact on circulating plasma cells from an open-label phase I dosage-escalation study. Arthritis Rheum. 62, 542–552 (2010).
Jacob, C. O., van der Meide, P. H. & McDevitt, H. O. In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to γ interferon. J. Exp. Med. 166, 798–803 (1987).
Summers, S. A. et al. Endogenous interleukin (IL)-17A promotes pristane-induced systemic autoimmunity and lupus nephritis induced by pristane. Clin. Exp. Immunol. 176, 341–350 (2014).
US National Libary of Science. ClinicalTrials.gov[online], (2014).
Martin, D. A. et al. A multiple dose study of AMG 811 (Anti-IFN-Gamma) in subjects with systemic lupus erythematosus and active nephritis. Ann. Rheum. Dis. 74 (Suppl. 2), 337 (2015).
Hoi, A. Y. et al. Macrophage migration inhibitory factor deficiency attenuates macrophage recruitment, glomerulonephritis, and lethality in MRL/lpr mice. J. Immunol. 177, 5687–5696 (2006).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Leng, L. et al. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 197, 1467–1476 (2003).
Djudjaj, S. et al. Macrophage migration inhibitory factor mediates proliferative GN via CD74. J. Am. Soc. Nephrol. http://dx.doi.org/10.1681/ASN.2015020149 (2015).
US National Libary of Science. ClinicalTrials.gov[online], (2015).
Kidney Disease Improving Global Outcomes. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. 2 (Suppl. 2), 240–242 (2012).
Falk, R. J. & Jennette, J. C. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N. Engl. J. Med. 318, 1651–1657 (1988).
Kain, R. et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat. Med. 14, 1088–1096 (2008).
Netzer, K. O. et al. The goodpasture autoantigen. Mapping the major conformational epitope(s) of α3(IV) collagen to residues 17–31 and 127–141 of the NC1 domain. J. Biol. Chem. 274, 11267–11274 (1999).
Lech, M. & Anders, H. J. The pathogenesis of lupus nephritis. J. Am. Soc. Nephrol. 24, 1357–1366 (2013).
Lockwood, C. M. et al. Treatment of refractory Wegener's granulomatosis with humanized monoclonal antibodies. QJM 89, 903–912 (1996).
US National Libary of Science. ClinicalTrials.gov[online], (2012).
US National Libary of Science. ClinicalTrials.gov[online], (2011).
Laurino, S., Chaudhry, A., Booth, A., Conte, G. & Jayne, D. Prospective study of TNFα blockade with adalimumab in ANCA-associated systemic vasculitis with renal involvement. Nephrol. Dial. Transplant. 25, 3307–3314 (2010).
Acknowledgements
Research in glomerular disease performed by S.R.H., P.Y.G., and A.R.K. is funded by project grants from the National Health and Medical Research Council of Australia (grant numbers 1048575, 1045065, 1046585, 1064112, and 1084869).
Author information
Authors and Affiliations
Contributions
A.R.K., P.Y.G., and S.R.H. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.R.K. has been a member of an advisory board for Roche Products, Australia. P.Y.G and S.R.H. declare no competing interests.
Rights and permissions
About this article
Cite this article
Holdsworth, S., Gan, PY. & Kitching, A. Biologics for the treatment of autoimmune renal diseases. Nat Rev Nephrol 12, 217–231 (2016). https://doi.org/10.1038/nrneph.2016.18
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2016.18
This article is cited by
-
RETRACTED ARTICLE: Transplantation of Mouse Induced Pluripotent Stem Cell-Derived Podocytes in a Mouse Model of Membranous Nephropathy Attenuates Proteinuria
Scientific Reports (2019)
-
Pulmorenales Syndrom
Der Nephrologe (2019)
-
Autocrine Tnf signaling favors malignant cells in myelofibrosis in a Tnfr2-dependent fashion
Leukemia (2018)
-
Progress in mechanisms and therapy for immunological kidney disease
Nature Reviews Nephrology (2018)
-
HLA and kidney disease: from associations to mechanisms
Nature Reviews Nephrology (2018)