Abstract
Genetic or autoimmune defects that lead to dysregulation of the alternative pathway of complement have been associated with the development of atypical haemolytic uraemic syndrome (aHUS), which is characterized by thrombocytopenia, haemolytic anaemia and acute kidney injury. The relationship between aHUS, podocyte dysfunction and the resultant proteinuria has not been adequately investigated. However, the report of mutations in diacylglycerol kinase ε (DGKE) as a cause of recessive infantile aHUS characterized by proteinuria, highlighted podocyte dysfunction as a potential complication of aHUS. DGKE deficiency was originally thought to trigger aHUS through pathogenetic mechanisms distinct from complement dysregulation; however, emerging findings suggest an interplay between DGKE and complement systems. Podocyte dysfunction with nephrotic-range proteinuria can also occur in forms of aHUS associated with genetic or autoimmune complement dysregulation without evidence of DGKE mutations. Furthermore, proteinuric glomerulonephritides can be complicated by aHUS, possibly as a consequence of podocyte dysfunction inducing endothelial injury and prothrombotic abnormalities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Noris, M. & Remuzzi, G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 361, 1676–1687 (2009).
Mele, C., Remuzzi, G. & Noris, M. Hemolytic uremic syndrome. Semin. Immunopathol. 36, 399–420 (2014).
Valoti, E. et al. A novel atypical hemolytic uremic syndrome-associated hybrid CFHR1/CFH gene encoding a fusion protein that antagonizes factor H-dependent complement regulation. J. Am. Soc. Nephrol. http://dx.doi.org/10.1681/ASN.2013121339.
Dragon-Durey, M. A. et al. Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 16, 555–563 (2005).
Noris, M., Mescia, F. & Remuzzi, G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. 8, 622–633 (2012).
Caprioli, J. et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108, 1267–1279 (2006).
Noris, M. et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin. J. Am. Soc. Nephrol. 5, 1844–1859 (2010).
Legendre, C. M. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013).
Zuber, J. et al. Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am. J. Transplant. 12, 3337–3354 (2012).
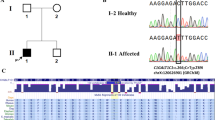
Lemaire, M. et al. Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat. Genet. 45, 531–536 (2013).
Orth, S. R. & Ritz, E. The nephrotic syndrome. N. Engl. J. Med. 338, 1202–1211 (1998).
Sanchez Chinchilla, D. et al. Complement Mutations in diacylglycerol kinase-epsilon-associated atypical hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. http://dx.doi.org/10.2215/CJN.01640214.
Westland, R. et al. Phenotypic expansion of DGKE-associated diseases. J. Am. Soc. Nephrol. 25, 1408–1414 (2014).
Remuzzi, G. & Ruggenenti, P. Thrombotic microangiopathies. in Renal Pathology (eds Tisher, C. & Brenner, B.) 1154–1184 (J. B. Lippincott, 1994).
Landau, D. et al. Familial hemolytic uremic syndrome associated with complement factor H deficiency. J. Pediatr. 138, 412–417 (2001).
Faul, C., Asanuma, K., Yanagida-Asanuma, E., Kim, K. & Mundel, P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 17, 428–437 (2007).
Barisoni, L., Schnaper, H. W. & Kopp, J. B. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin. J. Am. Soc. Nephrol 2, 529–542 (2007).
Ozaltin, F. et al. DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN. J. Am. Soc. Nephrol. 24, 377–384 (2013).
Rodriguez de Turco, E. B. et al. Diacylglycerol kinase epsilon regulates seizure susceptibility and long-term potentiation through arachidonoyl- inositol lipid signaling. Proc. Natl Acad. Sci. USA 98, 4740–4745 (2001).
Shulga, Y. V., Topham, M. K. & Epand, R. M. Regulation and functions of diacylglycerol kinases. Chem. Rev. 111, 6186–6208 (2011).
Lung, M. et al. Diacylglycerol kinase epsilon is selective for both acyl chains of phosphatidic acid or diacylglycerol. J. Biol. Chem. 284, 31062–31073 (2009).
Shulga, Y. V., Topham, M. K. & Epand, R. M. Substrate specificity of diacylglycerol kinase-epsilon and the phosphatidylinositol cycle. FEBS Lett. 585, 4025–4028 (2011).
Shulga, Y. V. et al. Molecular species of phosphatidylinositol-cycle intermediates in the endoplasmic reticulum and plasma membrane. Biochemistry 49, 312–317 (2010).
Rhee, S. G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70, 281–312 (2001).
Pettitt, T. R. et al. Diacylglycerol and phosphatidate generated by phospholipases C and D, respectively, have distinct fatty acid compositions and functions. Phospholipase D-derived diacylglycerol does not activate protein kinase C in porcine aortic endothelial cells. J. Biol. Chem. 272, 17354–17359 (1997).
Carew, M. A., Paleolog, E. M. & Pearson, J. D. The roles of protein kinase C and intracellular Ca2+ in the secretion of von Willebrand factor from human vascular endothelial cells. Biochem. J. 286, 631–635 (1992).
Ren, S., Shatadal, S. & Shen, G. X. Protein kinase C-beta mediates lipoprotein-induced generation of PAI-1 from vascular endothelial cells. Am. J. Physiol. Endocrinol. Metab. 278, E656–E662 (2000).
Herbert, J. M., Savi, P., Laplace, M. C., Dumas, A. & Dol, F. Chelerythrine, a selective protein kinase C inhibitor, counteracts pyrogen-induced expression of tissue factor without effect on thrombomodulin down-regulation in endothelial cells. Thromb. Res. 71, 487–493 (1993).
Chakraborti, T., Das, S. & Chakraborti, S. Proteolytic activation of protein kinase Calpha by peroxynitrite in stimulating cytosolic phospholipase A2 in pulmonary endothelium: involvement of a pertussis toxin sensitive protein. Biochemistry 44, 5246–5257 (2005).
Garcia, J. G., Stasek, J., Natarajan, V., Patterson, C. E. & Dominguez, J. Role of protein kinase C in the regulation of prostaglandin synthesis in human endothelium. Am. J. Respir. Cell. Mol. Biol. 6, 315–325 (1992).
Gomez, I., Foudi, N., Longrois, D. & Norel, X. The role of prostaglandin E2 in human vascular inflammation. Prostaglandins Leukot. Essent. Fatty Acids 89, 55–63 (2013).
Giannarelli, C., Zafar, M. U. & Badimon, J. J. Prostanoid and TP-receptors in atherothrombosis: is there a role for their antagonism? Thromb. Haemost. 104, 949–954 (2010).
Offermanns, S. Activation of platelet function through G protein-coupled receptors. Circ. Res. 99, 1293–1304 (2006).
Stegner, D. & Nieswandt, B. Platelet receptor signaling in thrombus formation. J. Mol. Med. (Berl.) 89, 109–121 (2011).
Nunn, D. L. & Watson, S. P. A diacylglycerol kinase inhibitor, R59022, potentiates secretion by and aggregation of thrombin-stimulated human platelets. Biochem. J. 243, 809–813 (1987).
Hofmann, T. et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263 (1999).
Winn, M. P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Moller, C. C. et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J. Am. Soc. Nephrol. 18, 29–36 (2007).
Krall, P. et al. Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS ONE 5, e12859 (2010).
Marie, J. et al. Hemolytic and uremic syndrome associated with an idiopathic nephrotic syndrome of 4 years' development [French]. Ann. Pediatr. (Paris) 16, 7–12 (1969).
Zhang, W. et al. Clinicopathological characteristics and outcome of Chinese patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: a 9-year retrospective study. Nephron Clin. Pract. 112, c177–c183 (2009).
Sinha, R., AlAbbas, A., Dionne, J. M. & Hurley, R. M. Simultaneous occurrence of atypical hemolytic uremic syndrome and acute lymphoblastic leukemia: a case report and literature review. Pediatr. Nephrol. 23, 835–839 (2008).
Kimura, M. et al. A patient with thrombotic microangiopathy accompanied by glomerular subendothelial electron dense deposits. Am. J. Nephrol. 18, 155–159 (1998).
Izumi, T. et al. An adult with acute poststreptococcal glomerulonephritis complicated by hemolytic uremic syndrome and nephrotic syndrome. Am. J. Kidney Dis. 46, e59–e63 (2005).
Jha, V. et al. Secondary membranoproliferative glomerulonephritis due to hemolytic uremic syndrome: an unusual presentation. Ren. Fail. 20, 845–850 (1998).
Alsina Segui, E., Martin Conde, M. L., Craver Hospital, L. & Fernandez Giraldez, E. Postpartum hemolytic uremic syndrome: a rare entity and a treatment challenge [Spanish]. Nefrologia 28, 120–121 (2008).
Nathanson, S., Ulinski, T., Fremeaux-Bacchi, V. & Deschenes, G. Secondary failure of plasma therapy in factor H deficiency. Pediatr. Nephrol. 21, 1769–1771 (2006).
Sinha, A. et al. Prompt plasma exchanges and immunosuppressive treatment improves the outcomes of anti-factor H autoantibody-associated hemolytic uremic syndrome in children. Kidney Int. 85, 1151–1160 (2014).
Sana, G. et al. Long-term remission of atypical HUS with anti-factor H antibodies after cyclophosphamide pulses. Pediatr. Nephrol. 29, 75–83 (2014).
Noone, D. et al. Successful treatment of DEAP-HUS with eculizumab. Pediatr. Nephrol. 29, 841–851 (2014).
Green, H. et al. Atypical HUS due to factor H antibodies in an adult patient successfully treated with eculizumab. Ren. Fail. 36, 1119–1121 (2014).
Cayci, F. S. et al. Eculizumab therapy in a child with hemolytic uremic syndrome and CFI mutation. Pediatr. Nephrol. 27, 2327–2331 (2012).
University College London FH aHUS mutation database [online], (2014).
Couser, W. G. Mediation of immune glomerular injury. J. Am. Soc. Nephrol. 1, 13–29 (1990).
Cybulsky, A. V., Quigg, R. J. & Salant, D. J. Experimental membranous nephropathy redux. Am. J. Physiol. Renal Physiol. 289, F660–F671 (2005).
Mathieson, P. W. What has the immune system got against the glomerular podocyte? Clin. Exp. Immunol. 134, 1–5 (2003).
Torbohm, I. et al. C5b-8 and C5b-9 modulate the collagen release of human glomerular epithelial cells. Kidney Int. 37, 1098–1104 (1990).
Chen, Z. H. et al. Triptolide reduces proteinuria in experimental membranous nephropathy and protects against C5b-9-induced podocyte injury in vitro. Kidney Int. 77, 974–988 (2010).
Pippin, J. W. et al. DNA damage is a novel response to sublytic complement C5b-9-induced injury in podocytes. J. Clin. Invest. 111, 877–885 (2003).
Ma, H., Sandor, D. G. & Beck, L. H. Jr. The role of complement in membranous nephropathy. Semin. Nephrol. 33, 531–542 (2013).
Lin, F., Emancipator, S. N., Salant, D. J. & Medof, M. E. Decay-accelerating factor confers protection against complement-mediated podocyte injury in acute nephrotoxic nephritis. Lab. Invest. 82, 563–569 (2002).
Alexander, J. J. et al. Mouse podocyte complement factor H: the functional analog to human complement receptor 1. J. Am. Soc. Nephrol 18, 1157–1166 (2007).
Lenderink, A. M. et al. The alternative pathway of complement is activated in the glomeruli and tubulointerstitium of mice with adriamycin nephropathy. Am. J. Physiol. Renal Physiol. 293, F555–F564 (2007).
Morita, Y. et al. Complement activation products in the urine from proteinuric patients. J. Am. Soc. Nephrol. 11, 700–707 (2000).
Onda, K. et al. Excretion of complement proteins and its activation marker C5b-9 in IgA nephropathy in relation to renal function. BMC Nephrol. 12, 64 (2011).
Endo, M. et al. Glomerular deposition and urinary excretion of complement factor H in idiopathic membranous nephropathy. Nephron Clin. Pract. 97, c147–c153 (2004).
Cybulsky, A. V., Papillon, J. & McTavish, A. J. Complement activates phospholipases and protein kinases in glomerular epithelial cells. Kidney Int. 54, 360–372 (1998).
Cybulsky, A. V., Takano, T., Papillon, J. & McTavish, A. J. Complement-induced phospholipase A2 activation in experimental membranous nephropathy. Kidney Int. 57, 1052–1062 (2000).
Panesar, M., Papillon, J., McTavish, A. J. & Cybulsky, A. V. Activation of phospholipase A2 by complement C5b-9 in glomerular epithelial cells. J. Immunol. 159, 3584–3594 (1997).
Takano, T. & Cybulsky, A. V. Complement C5b-9-mediated arachidonic acid metabolism in glomerular epithelial cells: role of cyclooxygenase-1 and -2. Am. J. Pathol. 156, 2091–2101 (2000).
Takano, T. et al. Inhibition of cyclooxygenases reduces complement-induced glomerular epithelial cell injury and proteinuria in passive Heymann nephritis. J. Pharmacol. Exp. Ther. 305, 240–249 (2003).
Cybulsky, A. V. et al. Complement C5b-9 membrane attack complex increases expression of endoplasmic reticulum stress proteins in glomerular epithelial cells. J. Biol. Chem. 277, 41342–41351 (2002).
Cybulsky, A. V. et al. The actin cytoskeleton facilitates complement-mediated activation of cytosolic phospholipase A2. Am. J. Physiol. Renal Physiol. 286, F466–F476 (2004).
Manenti, L. et al. Atypical haemolytic uraemic syndrome with underlying glomerulopathies. A case series and a review of the literature. Nephrol. Dial. Transplant. 28, 2246–2259 (2013).
Morita, S. et al. Hemolytic uremic syndrome associated with immunoglobulin A nephropathy: a case report and review of cases of hemolytic uremic syndrome with glomerular disease. Intern. Med. 38, 495–499 (1999).
Chang, A., Kowalewska, J., Smith, K. D., Nicosia, R. F. & Alpers, C. E. A clinicopathologic study of thrombotic microangiopathy in the setting of IgA nephropathy. Clin. Nephrol. 66, 397–404 (2006).
Krensky, A. M., Ingelfinger, J. R., Grupe, W. E. & Rosen, S. Hemolytic uremic syndrome and crescentic glomerulonephritis complicating childhood nephrosis. Clin. Nephrol. 19, 99–106 (1983).
Siegler, R. L., Brewer, E. D. & Pysher, T. J. Hemolytic uremic syndrome associated with glomerular disease. Am. J. Kidney Dis. 13, 144–147 (1989).
Sherbotie, J. R. et al. Hemolytic uremic syndrome associated with Denys–Drash syndrome. Pediatr. Nephrol. 14, 1092–1097 (2000).
Chen, G., Liu, H. & Liu, F. A glimpse of the glomerular milieu: from endothelial cell to thrombotic disease in nephrotic syndrome. Microvasc. Res. 89, 1–6 (2013).
Tkaczyk, M., Czupryniak, A., Owczarek, D., Lukamowicz, J. & Nowicki, M. Markers of endothelial dysfunction in children with idiopathic nephrotic syndrome. Am. J. Nephrol. 28, 197–202 (2008).
Isome, M. et al. Involvement of endothelial cell adhesion molecules in the development of anti-Thy-1 nephritis. Exp. Nephrol. 10, 338–347 (2002).
Gao, C. et al. Procoagulant activity of erythrocytes and platelets through phosphatidylserine exposure and microparticles release in patients with nephrotic syndrome. Thromb. Haemost. 107, 681–689 (2012).
Zoja, C. et al. Mesenchymal stem cell therapy promotes renal repair by limiting glomerular podocyte and progenitor cell dysfunction in adriamycin-induced nephropathy. Am. J. Physiol. Renal Physiol. 303, F1370–F1381 (2012).
Katavetin, P. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 359, 205–206; author reply 206–207 (2008).
Eremina, V. et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 358, 1129–1136 (2008).
Amore, A. et al. Aberrantly glycosylated IgA molecules downregulate the synthesis and secretion of vascular endothelial growth factor in human mesangial cells. Am. J. Kidney Dis. 36, 1242–1252 (2000).
Qin, W. et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J. Am. Soc. Nephrol. 22, 1137–1143 (2011).
Fogo, A. B. & Kon, V. The glomerulus-—a view from the inside—the endothelial cell. Int. J. Biochem. Cell Biol. 42, 1388–1397 (2010).
Wilkinson, G. F., Purkiss, J. R. & Boarder, M. R. The regulation of aortic endothelial cells by purines and pyrimidines involves co-existing P2y-purinoceptors and nucleotide receptors linked to phospholipase C. Br. J. Pharmacol. 108, 689–693 (1993).
Pueyo, M. E., N'Diaye, N. & Michel, J. B. Angiotensin II-elicited signal transduction via AT1 receptors in endothelial cells. Br. J. Pharmacol. 118, 79–84 (1996).
Brock, T. A. & Capasso, E. A. Thrombin and histamine activate phospholipase C in human endothelial cells via a phorbol ester-sensitive pathway. J. Cell Physiol. 136, 54–62 (1988).
Asselin, J. et al. A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase C gamma2 in platelets independent of the integrin α2β1. Blood 89, 1235–1242 (1997).
Keely, P. J. & Parise, L. V. The α2β1 integrin is a necessary co-receptor for collagen-induced activation of Syk and the subsequent phosphorylation of phospholipase Cγ2 in platelets. J. Biol. Chem. 271, 26668–26676 (1996).
Bhagyalakshmi, A., Berthiaume, F., Reich, K. M. & Frangos, J. A. Fluid shear stress stimulates membrane phospholipid metabolism in cultured human endothelial cells. J. Vasc. Res. 29, 443–449 (1992).
Anderson, M., Roshanravan, H., Khine, J. & Dryer, S. E. Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J. Cell Physiol. 229, 434–442 (2014).
Reiser, J., Sever, S. & Faul, C. Signal transduction in podocytes—spotlight on receptor tyrosine kinases. Nat. Rev. Nephrol. 10, 104–115 (2014).
Hong, M., Sandor, D. G. & Laurence H. B. The role of complement in membranous nephropathy. Semin. Nephrol. 33, 531–542 (2013).
Pickering, M. C. et al. C3 glomerulopathy: consensus report. Kidney Int. 84, 1079–1089 (2013).
Wang, Z. et al. NADPH oxidase-derived ROS contributes to upregulation of TRPC6 expression in puromycin aminonucleoside-induced podocyte injury. Cell Physiol. Biochem. 24, 619–626 (2009).
Kim, E. Y., Anderson, M. & Dryer, S. E. Insulin increases surface expression of TRPC6 channels in podocytes: role of NADPH oxidase and reactive oxygen species. Am. J. Physiol. Renal Physiol. 302, F298–F307 (2012).
Cybulsky, A. V., Papillon, J. & McTavish, A. J. Complement activates phospholipases and protein kinases in glomerular epithelial cells. Kidney Int. 54, 360–372 (1998).
Ren, G., Takano, T., Papillon, J. & Cybulsky, A. V. Cytosolic phospholipases A(2)-α enhances induction of endoplasmic reticulum stress. Biochim. Biophys. Acta 1803, 468–481 (2010).
Kim, E. Y., Anderson, M., Wilson, C., Hagmann, H., Benzing, T. & Dryer, S. E. NOX2 interacts with podocyte TRPC6 channels and contributes to their activation by diacylglycerol: essential role of podocin in formation of this complex: Am. J. Physiol. Cell Physiol. 305, C960–C971 (2013).
Jiang, L. et al. Over-expressing transient receptor potential cation channel 6 in podocytes induces cytoskeleton rearrangement through increases of intracellular Ca2+ and RhoA activation. Exp. Biol. Med. 236, 184–193 (2011).
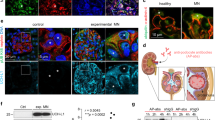
Bruneau, S. et al. Loss of DGKε induces endothelial cell activation and death independently of complement activation. Blood http://dx.doi.org/10.1182/blood-2014-06-579953.
Acknowledgements
The authors' work is supported by Fondazione ART per la Ricerca sui Trapianti ART ONLUS (Milano, Italy), the European Union Seventh Framework Programme FP7-EURenOmics project number 305608 and by Telethon (grant #GGP09075).
Author information
Authors and Affiliations
Contributions
M.N. and C.M researched data for the article. M.N. wrote the article. All authors contributed to discussion of the article's content and reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M.N. has received honoraria from Alexion Pharmaceuticals for giving lectures and participating on advisory boards. G.R. has consultancy agreements with AbbVie, Alexion Pharmaceuticals, Bayer Healthcare, Reata Pharmaceuticals, Novartis Pharma, AstraZeneca, Otsuka Pharmaceutical Europe and Concert Pharmaceuticals but receives no personal remuneration; compensations are instead paid to his institution for research and educational activities. C.M. declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Noris, M., Mele, C. & Remuzzi, G. Podocyte dysfunction in atypical haemolytic uraemic syndrome. Nat Rev Nephrol 11, 245–252 (2015). https://doi.org/10.1038/nrneph.2014.250
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2014.250
This article is cited by
-
Clinical features and outcomes of patients with diacylglycerol kinase epsilon nephropathy: a nationwide experience
Pediatric Nephrology (2023)
-
IgA nephropathy and atypical hemolytic uremic syndrome: a case series and a literature review
Journal of Nephrology (2022)
-
Atypical severe early-onset nephrotic syndrome: Answers
Pediatric Nephrology (2022)
-
Immune-complex glomerulonephritis with a membranoproliferative pattern in Frasier syndrome: a case report and review of the literature
BMC Nephrology (2020)
-
Whole exome sequencing revealed a novel homozygous variant in the DGKE catalytic domain: a case report of familial hemolytic uremic syndrome
BMC Medical Genetics (2020)