Key Points
-
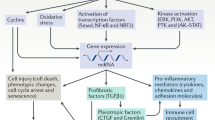
On the basis of promising preclinical and clinical data, the fatalistic concept of the 'point of no return' in chronic kidney disease (CKD) must be revised; evidence supports that CKD can be reversed
-
As fibrosis is controlled by overlapping pathways across all organs, the vast pipeline of antifibrotic drugs in preclinical and clinical development might be of benefit to patients with CKD in the future
-
Ongoing clinical trials to ameliorate fibrosis in the kidney are assessing transforming growth factor-β1 inhibitors, connective tissue growth factor inhibitors, bone morphogenic protein-7 agonists and endothelin-1 antagonists
-
Other strategies are exploring blockade of CC chemokine receptor type 2 and phosphodiesterase type 5 inhibition
Abstract
The concept of reversing chronic kidney disease (CKD) has been intensively researched over the past decade. Indeed, as the prevalence of end-stage renal disease is constantly on the rise, the lack of established antifibrotic therapies is a considerable unmet need in clinical practice. Now, the possibility of effective antifibrotic treatment has been established in experimental models of CKD and multiple antifibrotic compounds—in kidney disease, as well as in fibrotic diseases of the skin, liver and lung—are being assessed in clinical trials. These strategies target various components of the fibrotic pathway, from signalling molecules that include transforming growth factor-β, phosphatidylinositide 3-kinase and chemokines to microRNAs. Here, we discuss therapeutic concepts to inhibit or even reverse chronic kidney injury and review the leading candidate antifibrotic drugs to be introduced to clinical use.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ahlmén, J. Incidence of chronic renal insufficiency. A study of the incidence and pattern of renal insufficiency in adults during 1966–1971 in Gothenburg. Acta Med. Scand. Suppl. 582, 1–50 (1975).
Rutherford, W. E., Blondin, J., Miller, J. P., Greenwalt, A. S. & Vavra, J. D. Chronic progressive renal disease: rate of change of serum creatinine concentration. Kidney Int. 11, 62–70 (1977).
Leumann, E. P. Progression of renal insufficiency in pediatric patients: estimation from serum creatinine. Helv. Paediatr. Acta 33, 25–35 (1978).
Drawz, P. E., Goswami, P., Azem, R., Babineau, D. C. & Rahman, M. A simple tool to predict end-stage renal disease within 1 year in elderly adults with advanced chronic kidney disease. J. Am. Geriatr. Soc. 61, 762–768 (2013).
McLaughlin, M. J. & Courtney, A. E. Early recognition of CKD can delay progression. Practitioner 257, 13–17 (2013).
Whaley-Connell, A. T., Tamura, M. K., Jurkovitz, C. T., Kosiborod, M. & McCullough, P. A. Advances in CKD detection and determination of prognosis: executive summary of the National Kidney Foundation—Kidney Early Evaluation Program (KEEP) 2012 annual data report. Am. J. Kidney Dis. 61, S1–S3 (2013).
Fioretto, P., Steffes, M. W., Sutherland, D. E., Goetz, F. C. & Mauer, M. Reversal of lesions of diabetic nephropathy after pancreas transplantation, N. Engl. J. Med. 339, 69–75 (1998).
Fioretto, P., Sutherland, D. E., Najafian, B. & Mauer, M. Remodeling of renal interstitial and tubular lesions in pancreas transplant recipients. Kidney Int. 69, 907–912 (2006).
Remuzzi, A. et al. Regression of diabetic complications by islet transplantation in the rat. Diabetologia 52, 2653–2661 (2009).
Risdon, R. A., Sloper, J. C. & De Wardener, H. E. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2, 363–366 (1968).
Bohle, A., Glomb, D., Grund, K. E. & Mackensen, S. Correlation between relative interstitial volume of the renal cortex and serum creatinine concentration in minimal changes with nephrotic syndrome and in focal sclerosing glomerulonephritis. Virchows Arch. A Pathol. Anat. Histol. 376, 221–232 (1977).
Zeisberg, M. & Kalluri, R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am. J. Physiol. Cell Physiol. 304, C216–C225 (2013).
Wynn, T. A. & Ramalingam, T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18, 1028–1040 (2012).
Zeisberg, M. & Neilson, E. G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 21, 1819–1834 (2010).
Aydin, S. et al. Influence of microvascular endothelial cells on transcriptional regulation of proximal tubular epithelial cells. Am. J. Physiol. Cell Physiol. 294, C543–554 (2008).
Eddy, A. A. Molecular insights into renal interstitial fibrosis. J. Am. Soc. Nephrol. 7, 2495–2508 (1996).
Kim, H. et al. TIMP-1 deficiency does not attenuate interstitial fibrosis in obstructive nephropathy. J. Am. Soc. Nephrol. 12, 736–748 (2001).
Yang, J. et al. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J. Clin. Invest. 110, 1525–1538 (2002).
Zeisberg, M. et al. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease, PLoS Med. 3, e100 (2006).
Zeisberg, M. et al. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9, 964–968 (2003).
Zeisberg, M. et al. Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am. J. Physiol. Renal Physiol. 285, F1060–F1067 (2003).
Iredale, J. P. et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 102, 538–549 (1998).
Fallowfield, J. A. & Iredale, J. P. Reversal of liver fibrosis and cirrhosis—an emerging reality. Scott. Med. J. 49, 3–6 (2004).
Schuppan, D., Ruehl, M., Somasundaram, R. & Hahn, E. G. Matrix as a modulator of hepatic fibrogenesis, Semin. Liver Dis. 21, 351–372 (2001).
Barry-Hamilton, V. et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat. Med. 16, 1009–1017 (2010).
Zeisberg, M., Strutz, F. & Müller, G. A. Role of fibroblast activation in inducing interstitial fibrosis. J. Nephrol. 13 (Suppl. 3), S111–S120 (2000).
Harris, R. C. & Neilson, E. G. Toward a unified theory of renal progression. Annu. Rev. Med. 57, 365–380 (2006).
Rodemann, H. P. & Müller, G. A. Characterization of human renal fibroblasts in health and disease: II. In vitro growth, differentiation, and collagen synthesis of fibroblasts from kidneys with interstitial fibrosis. Am. J. Kidney Dis. 17, 684–686 (1991).
Bechtel, W. et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 16, 544–550 (2010).
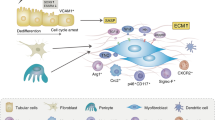
LeBleu, V. S. et al. Origin and function of myofibroblasts in kidney fibrosis. Nat. Med. 19, 1047–1053 (2013).
Iwano, M. et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110, 341–350 (2002).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Zeisberg, E. M., Potenta, S. E., Sugimoto, H., Zeisberg, M. & Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 19, 2282–2287 (2008).
Roberts, A. B. et al. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc. Natl Acad. Sci. USA 83, 4167–4171 (1986).
Richeldi, L. et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N. Engl. J. Med. 365, 1079–1087 (2011).
Frazier, K., Williams, S., Kothapalli, D., Klapper, H. & Grotendorst, G. R. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J. Invest. Dermatol. 107, 404–411 (1996).
Igarashi, A. et al. Connective tissue growth factor gene expression in tissue sections from localized scleroderma, keloid, and other fibrotic skin disorders. J. Invest. Dermatol. 106, 729–733 (1996).
Iwano, M. et al. Conditional abatement of tissue fibrosis using nucleoside analogs to selectively corrupt DNA replication in transgenic fibroblasts. Mol. Ther. 3, 149–159 (2001).
Hodgkins, K. S. & Schnaper, H. W. Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr. Nephrol. 27, 901–909 (2012).
Nangaku, M. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern. Med. 43, 9–17 (2004).
Zeisberg, M. & Neilson, E. G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 119, 1429–1437 (2009).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
Witek, R. P. et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology 50, 1421–1430 (2009).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Bussolati, B. et al. Isolation of renal progenitor cells from adult human kidney. Am. J. Pathol. 166, 545–555 (2005).
Maeshima, A., Yamashita, S. & Nojima, Y. Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J. Am. Soc. Nephrol. 14, 3138–3146 (2003).
Smeets, B. et al. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J. Pathol. 229, 645–659 (2013).
Kang, D. H. et al. Role of the microvascular endothelium in progressive renal disease. J. Am. Soc. Nephrol. 13, 806–816 (2002).
Matsumoto, M. et al. Hypoperfusion of peritubular capillaries induces chronic hypoxia before progression of tubulointerstitial injury in a progressive model of rat glomerulonephritis. J. Am. Soc. Nephrol. 15, 1574–1581 (2004).
Zoccali, C. Endothelial dysfunction in CKD: a new player in town? Nephrol. Dial. Transplant. 23, 783–785 (2008).
Maric-Bilkan, C., Flynn, E. R. & Chade, A. R. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am. J. Physiol. Renal Physiol. 302, F308–F315 (2012).
Chade, A. R. et al. Beneficial effects of antioxidant vitamins on the stenotic kidney. Hypertension 42, 605–612 (2003).
Pillebout, E. et al. Proliferation and remodeling of the peritubular microcirculation after nephron reduction: association with the progression of renal lesions. Am. J. Pathol. 159, 547–560 (2001).
Ohashi, R. et al. Peritubular capillary regression during the progression of experimental obstructive nephropathy. J. Am. Soc. Nephrol. 13, 1795–1805 (2002).
Clements, M. E., Chaber, C. J., Ledbetter, S. R. & Zuk, A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS ONE 8, e70464 (2013).
Renkonen, R., Turunen, J. P., Rapola, J. & Häyry, P. Characterization of high endothelial-like properties of peritubular capillary endothelium during acute renal allograft rejection. Am. J. Pathol. 137, 643–651 (1990).
Chade, A. R. & Kelsen, S. Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: a novel potential therapeutic approach. Am. J. Physiol. Renal Physiol. 302, F1342–F1350 (2012).
Sun, D. et al. Thrombospondin-1 short hairpin RNA suppresses tubulointerstitial fibrosis in the kidney of ureteral obstruction by ameliorating peritubular capillary injury. Kidney Blood Press. Res. 35, 35–47 (2012).
Yamamoto, Y. et al. Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes 53, 1831–1840 (2004).
Ichinose, K. et al. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes 54, 2891–2903 (2005).
Chade, A. R. Renovascular disease, microcirculation, and the progression of renal injury: role of angiogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R783–R790 (2011).
Pergola, P. E. et al. Effect of bardoxolone methyl on kidney function in patients with T2D and Stage 3b–4 CKD. Am. J. Nephrol. 33, 469–476 (2011).
Lagaaij, E. L. et al. Endothelial cell chimerism after renal transplantation and vascular rejection. Lancet 357, 33–37 (2001).
Grimm, P. C. et al. Neointimal and tubulointerstitial infiltration by recipient mesenchymal cells in chronic renal-allograft rejection. N. Engl. J. Med. 345, 93–97 (2001).
Fadini, G. P., Losordo, D. & Dimmeler, S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 110, 624–637 (2012).
Kurts, C., Panzer, U., Anders, H. J. & Rees, A. J. The immune system and kidney disease: basic concepts and clinical implications. Nat. Rev. Immunol. 13, 738–753 (2013).
Anders, H. J. et al. Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRL-Fas(lpr) mice. J. Am. Soc. Nephrol. 15, 1504–1513 (2004).
Ricardo, S. D., van Goor, H. & Eddy, A. A. Macrophage diversity in renal injury and repair. J. Clin. Invest. 118, 3522–3530 (2008).
François, A. et al. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res. Ther. 15, R168 (2013).
Huaux, F. et al. Eosinophils and T lymphocytes possess distinct roles in bleomycin-induced lung injury and fibrosis. J. Immunol. 171, 5470–5481 (2003).
Lebleu, V. S., Sugimoto, H., Miller, C. A., Gattone, V. H. 2nd & Kalluri, R. Lymphocytes are dispensable for glomerulonephritis but required for renal interstitial fibrosis in matrix defect-induced Alport renal disease, Lab. Invest. 88, 284–292 (2008).
Kaviratne, M. et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-β independent. J. Immunol. 173, 4020–4029 (2004).
Elger, M. et al. Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J. Am. Soc. Nephrol. 14, 1506–1518 (2003).
Little, M. H. Regrow or repair: potential regenerative therapies for the kidney. J. Am. Soc. Nephrol. 17, 2390–2401 (2006).
Lindoso, R. S., Verdoorn, K. S. & Einicker-Lamas, M. Renal recovery after injury: the role of Pax-2. Nephrol. Dial. Transplant. 24, 2628–2633 (2009).
Narlis, M., Grote, D., Gaitan, Y., Boualia, S. K. & Bouchard, M. Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney, J. Am. Soc. Nephrol. 18, 1121–1129 (2007).
Park, J. S., Valerius, M. T. & McMahon, A. P. Wnt/β-catenin signaling regulates nephron induction during mouse kidney development. Development 134, 2533–2539 (2007).
Bramlage, C. P. et al. Bone morphogenetic protein (BMP)-7 expression is decreased in human hypertensive nephrosclerosis. BMC Nephrol. 11, 31 (2010).
Dudley, A. T., Lyons, K. M. & Robertson, E. J. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 9, 2795–2807 (1995).
Vukicevic, S. et al. Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J. Clin. Invest. 102, 202–214 (1998).
Sugimoto, H., Grahovac, G., Zeisberg, M. & Kalluri, R. Renal fibrosis and glomerulosclerosis in a new mouse model of diabetic nephropathy and its regression by bone morphogenic protein-7 and advanced glycation end product inhibitors. Diabetes 56, 1825–1833 (2007).
Morrissey, J. et al. Bone morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J. Am. Soc. Nephrol. 13 (Suppl. 1), S14–S21 (2002).
Zeisberg, M., Shah, A. A. & Kalluri, R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J. Biol. Chem. 280, 8094–8100 (2005).
Swencki-Underwood, B. et al. Expression and characterization of a human BMP-7 variant with improved biochemical properties. Protein Expr. Purif. 57, 312–319 (2008).
Sugimoto, H. et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat. Med. 18, 396–404 (2012).
Bachmann, S., Kriz, W., Kuhn, C. & Franke, W. W. Differentiation of cell types in the mammalian kidney by immunofluorescence microscopy using antibodies to intermediate filament proteins and desmoplakins. Histochemistry 77, 365–394 (1983).
Ebrahimi, B. et al. Mesenchymal stem cells improve medullary inflammation and fibrosis after revascularization of swine atherosclerotic renal artery stenosis. PLoS ONE 8, e67474 (2013).
Ebrahimi, B. et al. Addition of endothelial progenitor cells to renal revascularization restores medullary tubular oxygen consumption in swine renal artery stenosis. Am. J. Physiol. Renal Physiol. 302, F1478–F1485 (2012).
Reinders, M. E. et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: results of a phase I study. Stem Cells Transl. Med. 2, 107–111 (2013).
Tan, J. et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA 307, 1169–1177 (2012).
Didié, M. et al. Parthenogenetic stem cells for tissue-engineered heart repair. J. Clin. Invest. 123, 1285–1298 (2013).
Bhutani, N., Burns, D. M. & Blau, H. M. DNA demethylation dynamics. Cell 146, 866–872 (2011).
Van Beneden, K. et al. Comparison of trichostatin A and valproic acid treatment regimens in a mouse model of kidney fibrosis. Toxicol. Appl. Pharmacol. 271, 276–284 (2013).
Manson, S. R., Song, J. B., Hruska, K. A. & Austin, P. F. HDAC dependent transcriptional repression of Bmp-7 potentiates TGF-β mediated renal fibrosis in obstructive uropathy. J. Urol. 191, 242–252 (2013).
Reddy, M. A. et al. Losartan reverses permissive epigenetic changes in renal glomeruli of diabetic db/db mice. Kidney Int. http://dx.doi.org/10.1038/ki.2013.387 (2013).
Tampe, B. et al. Tet3-mediated hydroxymethylation of epigenetically silenced genes contributes to bone morphogenic protein 7-induced reversal of kidney fibrosis. J. Am. Soc. Nephrol. (in press).
Kolfschoten, I. G. et al. A genetic screen identifies PITX1 as a suppressor of RAS activity and tumorigenicity. Cell 121, 849–858 (2005).
Suzuki, T. & Miyata, N. Non-hydroxamate histone deacetylase inhibitors. Curr. Med. Chem. 12, 2867–2880 (2005).
Israili, Z. H. et al. The disposition and pharmacokinetics in humans of 5-azacytidine administered intravenously as a bolus or by continuous infusion. Cancer Res. 36, 1453–1461 (1976).
Chen, J. et al. The metabolic syndrome and chronic kidney disease in US adults. Ann. Intern. Med. 140, 167–174 (2004).
Molnar, M. Z. et al. ACE inhibitor and angiotensin receptor blocker use and mortality in patients with chronic kidney disease. J. Am. Coll. Cardiol. http://dx.doi.org/10.1016/j.jacc.2013.10.050 (2013).
Kilbride, H. S. et al. Accuracy of the MDRD (modification of diet in renal disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am. J. Kidney Dis. 61, 57–66 (2013).
Stengel, B. et al. The French Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort study. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gft388 (2013).
Gabbai, F. B. et al. Relationship between ambulatory BP and clinical outcomes in patients with hypertensive CKD. Clin. J. Am. Soc. Nephrol. 7, 1770–1776 (2012).
Kanasaki, M., Nagai, T., Kitada, M., Koya, D. & Kanasaki, K. Elevation of the antifibrotic peptide N-acetyl-seryl-aspartyl-lysyl-proline: a blood pressure-independent beneficial effect of angiotensin I-converting enzyme inhibitors. Fibrogenesis Tissue Repair 4, 25 (2011).
Collins, A. J. et al. US Renal Data System Annual Data Report. Am. J. Kidney Dis. 59 (Suppl. 1), evii (2011).
Zeisberg, M. & Müller, G. A. Mechanistic insights into the antifibrotic activity of aliskiren in the kidney, Hypertens. Res. 35, 266–268 (2012).
Nguyen, G. et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 109, 1417–1427 (2002).
Feldman, D. L. et al. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension 52, 130–136 (2008).
Messerli, F. H. & Bangalore, S. ALTITUDE trial and dual RAS blockade: the alluring but soft science of the surrogate end point. Am. J. Med. 126, e1–e3 (2013).
Lecaire, T. J., Klein, B. E., Howard, K. P., Lee, K. E. & Klein, R. Risk for end-stage renal disease over 25 years in the population-based WESDR cohort. Diabetes Care 37, 381–388 (2013).
Border, W. A., Okuda, S., Languino, L. R., Sporn, M. B. & Ruoslahti, E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor β1. Nature 346, 371–374 (1990).
Border, W. A. et al. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature 360, 361–364 (1992).
Isaka, Y. et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat. Med. 2, 418–423 (1996).
US National Library of Medicine. ClinicalTrials.gov[online], (2010).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
Garber, K. Companies waver in efforts to target transforming growth factor β in cancer. J. Natl Cancer Inst. 101, 1664–1667 (2009).
Akhurst, R. J. TGF-β antagonists: why suppress a tumor suppressor? J. Clin. Invest. 109, 1533–1536 (2002).
Massagué, J. How cells read TGF-β signals. Nat. Rev. Mol. Cell Biol. 1, 169–178 (2000).
Zeisberg, M. et al. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J. Biol. Chem. 282, 23337–23347 (2007).
Hruska, K. A. et al. Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am. J. Physiol. Renal Physiol. 279, F130–F143 (2000).
Wang, S. & Hirschberg, R. BMP7 antagonizes TGF-β-dependent fibrogenesis in mesangial cells. Am. J. Physiol. Renal Physiol. 284, F1006–F1013 (2003).
Zeisberg, E. M. et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 13, 952–961 (2007).
Flier, S. N. et al. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J. Biol. Chem. 285, 20202–20212 (2010).
Bradham, D. M., Igarashi, A., Potter, R. L. & Grotendorst, G. R. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J. Cell Biol. 114, 1285–1294 (1991).
Nguyen, T. Q. et al. Plasma connective tissue growth factor is an independent predictor of end-stage renal disease and mortality in type 1 diabetic nephropathy. Diabetes Care 31, 1177–1182 (2008).
Yokoi, H. et al. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 15, 1430–1440 (2004).
Guha, M., Xu, Z. G., Tung, D., Lanting, L. & Natarajan, R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 21, 3355–3368 (2007).
Adler, S. G. et al. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin. J. Am. Soc. Nephrol. 5, 1420–1428 (2010).
Taniguchi, H. et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur. Respir. J. 35, 821–829 (2010).
Shimizu, T. et al. Pirfenidone improves renal function and fibrosis in the post-obstructed kidney. Kidney Int. 54, 99–109 (1998).
Ziyadeh, F. N. et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-β antibody in db/db diabetic mice. Proc. Natl Acad. Sci. USA 97, 8015–8020 (2000).
RamachandraRao, S. P. et al. Pirfenidone is renoprotective in diabetic kidney disease, J. Am. Soc. Nephrol. 20, 1765–1775 (2009).
Cho, M. E., Smith, D. C., Branton, M. H., Penzak, S. R. & Kopp, J. B. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2, 906–913 (2007).
Sharma, K. et al. Pirfenidone for diabetic nephropathy. J. Am. Soc. Nephrol. 22, 1144–1151 (2011).
Charo, I. F. & Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Sarvaiya, P. J., Guo, D., Ulasov, I., Gabikian, P. & Lesniak, M. S. Chemokines in tumor progression and metastasis. Oncotarget 4, 2171–2185 (2013).
Anders, H. J., Vielhauer, V. & Schlondorff, D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 63, 401–415 (2003).
Reich, B. et al. Fibrocytes develop outside the kidney but contribute to renal fibrosis in a mouse model. Kidney Int. 84, 78–89 (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
Sullivan, T. et al. CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am. J. Physiol. Renal Physiol. 305, F1288–F1297 (2013).
Hanefeld, M. et al. Orally-administered chemokine receptor CCR2 antagonist CCX140-B in type 2 diabetes: a pilot double-blind, randomized clinical trial. J. Diabetes Metab. 3, 225 (2012).
Inoue, A. et al. The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. J. Biol. Chem. 264, 14954–14959 (1989).
Arinami, T. et al. Chromosomal assignments of the human endothelin family genes: the endothelin-1 gene (EDN1) to 6p23-p24, the endothelin-2 gene (EDN2) to 1p34, and the endothelin-3 gene (EDN3) to 20q13.2-q13.3. Am. J. Hum. Genet. 48, 990–996 (1991).
Karet, F. E. Endothelin peptides and receptors in human kidney, Clin. Sci. (Lond.) 91, 267–273 (1996).
Dhaun, N., Goddard, J. & Webb, D. J. The endothelin system and its antagonism in chronic kidney disease J. Am. Soc. Nephrol. 17, 943–955 (2006).
Attinà, T., Camidge, R., Newby, D. E. & Webb, D. J. Endothelin antagonism in pulmonary hypertension, heart failure, and beyond. Heart 91, 825–831 (2005).
Wesson, D. E., Simoni, J. & Green, D. F. Reduced extracellular pH increases endothelin-1 secretion by human renal microvascular endothelial cells. J. Clin. Invest. 101, 578–583 (1998).
Hocher, B. & Paul, M. Transgenic animal models for the analysis of the renal endothelin system. Nephrol. Dial. Transplant. 15, 935–937 (2000).
Saito, Y. et al. Application of monoclonal antibodies for endothelin to hypertensive research. Hypertension 15, 734–738 (1990).
Kuc, R. & Davenport, A. P. Comparison of endothelin-A and endothelin-B receptor distribution visualized by radioligand binding versus immunocytochemical localization using subtype selective antisera. J. Cardiovasc. Pharmacol. 44 (Suppl. 1), S224–S226 (2004).
Benigni, A. et al. A specific endothelin subtype A receptor antagonist protects against injury in renal disease progression. Kidney Int. 44, 440–444 (1993).
Opocenský, M. et al. Late-onset endothelin-A receptor blockade reduces podocyte injury in homozygous Ren-2 rats despite severe hypertension. Hypertension 48, 965–971 (2006).
Boffa, J. J., Tharaux, P. L., Dussaule, J. C. & Chatziantoniou, C. Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension 37, 490–496 (2001).
Gómez-Garre, D. et al. Effects and interactions of endothelin-1 and angiotensin II on matrix protein expression and synthesis and mesangial cell growth. Hypertension 27, 885–892 (1996).
Fukuda, K. et al. Role of endothelin as a mitogen in experimental glomerulonephritis in rats, Kidney Int. 49, 1320–1329 (1996).
Benigni, A. et al. Renoprotective effect of contemporary blocking of angiotensin II and endothelin-1 in rats with membranous nephropathy. Kidney Int. 54, 353–359 (1998).
Saleh, M. A., Pollock, J. S. & Pollock, D. M. Distinct actions of endothelin A-selective versus combined endothelin A/B receptor antagonists in early diabetic kidney disease. J. Pharmacol. Exp. Ther. 338, 263–270 (2011).
Wenzel, R. R. et al. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J. Am. Soc. Nephrol. 20, 655–664 (2009).
Mann, J. F. et al. Avosentan for overt diabetic nephropathy. J. Am. Soc. Nephrol. 21, 527–535 (2010).
Chen, Y. M., Wu, K. D., Tsai, T. J. & Hsieh, B. S. Pentoxifylline inhibits PDGF-induced proliferation of and TGF-β-stimulated collagen synthesis by vascular smooth muscle cells. J. Mol. Cell Cardiol. 31, 773–783 (1999).
Strutz, F. et al. Effects of pentoxifylline, pentifylline and gamma-interferon on proliferation, differentiation, and matrix synthesis of human renal fibroblasts. Nephrol. Dial. Transplant. 15, 1535–1546 (2000).
Lin, S. L. et al. Pentoxifylline attenuated the renal disease progression in rats with remnant kidney. J. Am. Soc. Nephrol. 13, 2916–2929 (2002).
Rodriguez-Morán, M. et al. Effects of pentoxifylline on the urinary protein excretion profile of type 2 diabetic patients with microproteinuria: a double-blind, placebo-controlled randomized trial. Clin. Nephrol. 66, 3–10 (2006).
Perkins, R. M. et al. Effect of pentoxifylline on GFR decline in CKD: a pilot, double-blind, randomized, placebo-controlled trial. Am. J. Kidney Dis. 53, 606–616 (2009).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
Zalba, G. et al. Is the balance between nitric oxide and superoxide altered in spontaneously hypertensive rats with endothelial dysfunction? Nephrol. Dial. Transplant. 16 (Suppl. 1), 2–5 (2001).
Firoozi, F., Longhurst, P. A. & White, M. D. In vivo and in vitro response of corpus cavernosum to phosphodiesterase-5 inhibition in the hypercholesterolaemic rabbit. BJU Int. 96, 164–168 (2005).
Dousa, T. P. Cyclic-3′,5′-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int. 55, 29–62 (1999).
Lau, D. H., Mikhailidis, D. P. & Thompson, C. S. The effect of vardenafil (a PDE type 5 inhibitor) on renal function in the diabetic rabbit: a pilot study. In Vivo 21, 851–854 (2007).
Jeong, K. H. et al. Effects of sildenafil on oxidative and inflammatory injuries of the kidney in streptozotocin-induced diabetic rats. Am. J. Nephrol. 29, 274–282 (2009).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
Wilcox, C. S. Reactive oxygen species: roles in blood pressure and kidney function. Curr. Hypertens. Rep. 4, 160–166 (2002).
Touyz, R. M. Reactive oxygen species in vascular biology: role in arterial hypertension. Expert Rev. Cardiovasc. Ther. 1, 91–106 (2003).
Vaziri, N. D. & Rodriguez-Iturbe, B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2, 582–593 (2006).
Mueller, C. F., Laude, K., McNally, J. S. & Harrison, D. G. ATVB in focus: redox mechanisms in blood vessels, Arterioscler. Thromb. Vasc. Biol. 25, 274–278 (2005).
Griendling, K. K. NADPH oxidases: new regulators of old functions. Antioxid. Redox Signal. 8, 1443–1445 (2006).
Lassegue, B. & Clempus, R. E. Vascular NAD(P)H oxidases: specific features, expression, and regulation, Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R277–R297 (2003).
Rajagopalan, S. et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 97, 1916–1923 (1996).
Virdis, A., Neves, M. F., Amiri, F., Touyz, R. M. & Schiffrin, E. L. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J. Hypertens. 22, 535–542 (2004).
Aoyama, T. et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56, 2316–2327 (2012).
Fortuño, A. et al. Association of increased phagocytic NADPH oxidase-dependent superoxide production with diminished nitric oxide generation in essential hypertension. J. Hypertens. 22, 2169–2175 (2004).
Higashi, Y. et al. Endothelial function and oxidative stress in renovascular hypertension. N. Engl. J. Med. 346, 1954–1962 (2002).
Lip, G. Y. et al. Oxidative stress in malignant and non-malignant phase hypertension. J. Hum. Hypertens. 16, 333–336 (2002).
Acknowledgements
M.Z. is supported by German Research Foundation (DFG) grants ZE523/2-1 and ZE523/3-1.
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed its content, wrote the manuscript and edited it prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tampe, D., Zeisberg, M. Potential approaches to reverse or repair renal fibrosis. Nat Rev Nephrol 10, 226–237 (2014). https://doi.org/10.1038/nrneph.2014.14
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2014.14
This article is cited by
-
Pirfenidone alleviates fibrosis by acting on tumour–stroma interplay in pancreatic cancer
British Journal of Cancer (2024)
-
Chitosan oligosaccharide alleviates renal fibrosis through reducing oxidative stress damage and regulating TGF-β1/Smads pathway
Scientific Reports (2022)
-
A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-β signaling
Nature Communications (2022)
-
RCAN1.4 attenuates renal fibrosis through inhibiting calcineurin-mediated nuclear translocation of NFAT2
Cell Death Discovery (2021)
-
Chronic treatment with the (iso-)glutaminyl cyclase inhibitor PQ529 is a novel and effective approach for glomerulonephritis in chronic kidney disease
Naunyn-Schmiedeberg's Archives of Pharmacology (2021)