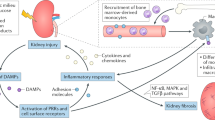
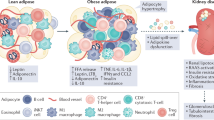
Abstract
Many lines of evidence, ranging from in vitro experiments and pathological examinations to epidemiological studies, show that inflammation is a cardinal pathogenetic mechanism in diabetic nephropathy. Thus, modulation of inflammatory processes in the setting of diabetes mellitus is a matter of great interest for researchers today. The relationships between inflammation and the development and progression of diabetic nephropathy involve complex molecular networks and processes. This Review, therefore, focuses on key proinflammatory molecules and pathways implicated in the development and progression of diabetic nephropathy: the chemokines CCL2, CX3CL1 and CCL5 (also known as MCP-1, fractalkine and RANTES, respectively); the adhesion molecules intercellular adhesion molecule 1, vascular cell adhesion protein 1, endothelial cell-selective adhesion molecule, E-selectin and α-actinin 4; the transcription factor nuclear factor κB; and the inflammatory cytokines IL-1, IL-6, IL-18 and tumor necrosis factor. Advances in the understanding of the roles that these inflammatory pathways have in the context of diabetic nephropathy will facilitate the discovery of new therapeutic targets. In the next few years, promising new therapeutic strategies based on anti-inflammatory effects could be successfully translated into clinical treatments for diabetic complications, including diabetic nephropathy.
Key Points
-
A wide range of proinflammatory molecules and pathways participate in the pathophysiological spectrum of diabetic nephropathy, including proinflammatory cytokines, chemokines and their receptors, adhesion molecules and transcription factors
-
Inflammatory mechanisms are important in the pathophysiology of diabetic nephropathy and explain how metabolic and hemodynamic abnormalities in patients with diabetes mellitus translate to functional and structural kidney injury
-
IL-18 is strongly associated with diabetic nephropathy; levels of this proinflammatory cytokine might be useful as an early marker of renal dysfunction in patients with type 2 diabetes mellitus
-
Abnormal C-C motif chemokine 2 signaling results in reorganization of the actin cytoskeleton and downregulation of nephrin, causing changes in podocyte structure and function that are associated with albuminuria
-
Endothelial cell-selective adhesion molecule (ESAM) is linked to diabetic nephropathy: high blood levels of glucose decrease ESAM expression, causing tight junction expansion, decreased endothelial fenestration and albuminuria
-
Inhibition of tumor necrosis factor using pentoxifylline improves markers of glomerular and tubulointerstitial injury in patients with diabetic nephropathy; ongoing randomized, controlled clinical trials are investigating the renoprotective effects of this agent
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Cooper, M. E. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia 44, 1957–1972 (2001).
Wolf, G. New insights into the pathophysiology of diabetic nephropathy: from haemodynamics to molecular pathology. Eur. J. Clin. Invest. 34, 785–796 (2004).
Martini, S., Eichinger, F., Nair, V. & Kretzler, M. Defining human diabetic nephropathy on the molecular level: integration of transcriptomic profiles with biological knowledge. Rev. Endocr. Metab. Disord. 9, 267–274 (2008).
Zandi-Nejad, K., Eddy, A. A., Glassock, R. J. & Brenner, B. M. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int. Suppl. 92, S76–S89 (2004).
Abbate, M., Zoja, C. & Remuzzi, G. How does proteinuria cause progressive renal damage? J. Am. Soc. Nephrol. 17, 2974–2984 (2006).
Vilcek, J. in The Cytokine Handbook 4th edn (eds Thomson, A. W. & Lotze, M. T.) 3–18 (Academic Press, London, 2003).
Alexandraki, K. et al. Inflammatory process in type 2 diabetes. The role of cytokines. Ann. NY Acad. Sci. 1084, 89–117 (2006).
Hasegawa, G. et al. Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 40, 1007–1012 (1991).
Hasegawa, G., Nakano, K. & Kondo, M. Role of TNF and IL-1 in the development of diabetic nephropathy. Nefrologia 15, 1–4 (1995).
Nakamura, T. et al. mRNA expression of growth factors in glomeruli from diabetic rats. Diabetes 42, 450–456 (1993).
Sugimoto, H., Shikata, K., Wada, J., Horiuchi, S. & Makino, H. Advanced glycation end products-cytokine-nitric oxide sequence pathway in the development of diabetic nephropathy: aminoguanidine ameliorates the overexpression of tumour necrosis factor-α and inducible oxide synthase in diabetic rat glomeruli. Diabetologia 42, 878–886 (1999).
Navarro, J. F. & Mora, C. Role of inflammation in diabetic complications. Nephrol. Dial. Transplant. 20, 2601–2604 (2005).
Navarro-González, J. F. & Mora-Fernández, C. The role of inflammatory cytokines in diabetic nephropathy. J. Am. Soc. Nephrol. 19, 433–442 (2008).
Sassy-Prigent, C. et al. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes 49, 466–475 (2000).
Navarro, J. F. et al. Tumour necrosis factor-α gene expression in diabetic nephropathy: relationship with urinary albumin excretion and effect of angiotensin-converting enzyme inhibition. Kidney Int. Suppl. 99, S98–S102 (2005).
Navarro, J. F., Milena, F. J., Mora, C., León, C. & García, J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am. J. Nephrol. 26, 562–570 (2006).
Pfeilschifter, J., Pignat, W., Vosbeck, K. & Märki, F. Interleukin 1 and tumor necrosis factor synergistically stimulate prostaglandin synthesis and phospholipase A2 release from rat renal mesangial cells. Biochem. Biophys. Res. Commun. 159, 385–394 (1989).
Pfeilschifter, J. & Mühl, H. Interleukin-1 and tumor necrosis factor potentiate angiotensin II- and calcium ionophore-stimulated prostaglandin E2 synthesis in rat renal mesangial cells. Biochem. Biophys. Res. Commun. 169, 585–595 (1990).
Jones, S., Jones, S. & Phillips, A. O. Regulation of renal proximal tubular epithelial cell hyaluronan generation: implications for diabetic nephropathy. Kidney Int. 59, 1739–1749 (2001).
Royall, J. A. et al. Tumor necrosis factor and interleukin 1 increase vascular endothelial permeability. Am. J. Physiol. 257, L339–L410 (1989).
Suzuki, D. et al. In situ hybridization of interleukin 6 in diabetic nephropathy. Diabetes 44, 1233–1238 (1995).
Coleman, D. L. & Ruef, C. Interleukin-6: an autocrine regulator of mesangial cell growth. Kidney Int. 41, 604–606 (1992).
Nosadini, R. et al. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes 49, 476–484 (2000).
Dalla Vestra, M. et al. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J. Am. Soc. Nephrol. 16 (Suppl. 1), S78–S82 (2005).
Sekizuka, K. et al. Detection of serum IL-6 in patients with diabetic nephropathy. Nephron 68, 284–285 (1994).
Thomson, S. C. et al. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J. Clin. Invest. 107, 217–224 (2001).
Melnikov, V. Y. et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J. Clin. Invest. 107, 1145–1152 (2001).
Melnikov, V. Y. et al. Neutrophil-independent mechanisms of caspase-1 and IL-18 mediated ischemic acute tubular necrosis in mice. J. Clin. Invest. 110, 1083–1091 (2002).
Miyauchi, K., Takiyama, Y., Honjyo, J., Tatano, M. & Haneda, M. Upregulated IL-18 expression in type 2 diabetic subjects with nephropathy: TGF-β1 enhanced IL-18 expression in human renal proximal tubular epithelial cells. Diabetes Res. Clin. Pract. 83, 190–199 (2009).
Okamura, H. et al. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature 378, 88–91 (1995).
Dai, S. M., Matsuno, H., Nakamura, H., Nishioka, K. & Yudoh, K. Interleukin-18 enhances monocyte tumor necrosis factor α and interleukin-1β production induced by direct contact with T lymphocytes: implications in rheumatoid arthritis. Arthritis Rheum. 50, 432–443 (2004).
Mariño, E. & Cardier, J. E. Differential effects of IL-18 on endothelial cell apoptosis mediated by TNF-α and Fas (CD59). Cytokine 22, 142–148 (2003).
Stuyt, R. J. et al. Selective regulation of intercellular adhesion molecule-1 expression by interleukin-18 and interleukin-12 on human monocytes. Immunology 110, 329–334 (2003).
Moriwaki, Y. et al. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism 52, 605–608 (2003).
Mahmoud, R. A., el-Ezz, S. A. & Hegazy, A. S. Increased serum levels of interleukin-18 in patients with diabetic nephropathy. Ital. J. Biochem. 53, 73–81 (2004).
Nakamura, A. et al. Serum interleukin-18 levels are associated with nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Care 28, 2890–2895 (2005).
Wong, C. K. et al. Aberrant activation profile of cytokines and mitogen-activated protein kinases in type 2 diabetic patients with nephropathy. Clin. Exp. Immunol. 149, 123–131 (2007).
Araki, S. et al. Predictive impact of elevated serum level of IL-18 for early renal dysfunction in type 2 diabetes: an observational follow-up study. Diabetologia 50, 867–873 (2007).
Fujita, T. et al. Interleukin-18 contributes more closely to the progression of diabetic nephropathy than other diabetic complications. Acta Diabetol. doi:10.1007/s00592-010-0178-4.
Wang, H., Czura, C. J. & Tracey, K. J. in The Cytokine Handbook 4th edn (eds Thomson, A. W. & Lotze, M. T.) 837–860 (Academic Press, London, 2003).
Ortiz, A. et al. Involvement of tumor necrosis factor-alpha in the pathogenesis of experimental and human glomerulonephritis. Adv. Nephrol. Necker Hosp. 24, 53–77 (1995).
Bertani, T. et al. Tumor necrosis factor induces glomerular damage in rabbit. Am. J. Pathol. 134, 419–430 (1989).
Laster, S. M., Wood, J. G. & Gooding, L. R. Tumor necrosis factor can induce both apoptotic and necrotic forms of cell lysis. J. Immunol. 141, 2629–2634 (1988).
Baud, L., Pérez, J., Friedlander, G. & Ardaillou, R. Tumor necrosis factor stimulates prostaglandin production and cyclic AMP levels in rat cultured mesangial cells. FEBS Lett. 239, 50–54 (1998).
Wójciak-Stothard, B., Entwistle, A., Garg, R. & Ridley, A. J. Regulation of TNF-α-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J. Cell. Physiol. 176, 150–165 (1998).
Koike, N., Takamura, T. & Kaneko, S. Induction of reactive oxygen species from isolated rat glomerli by protein kinase C activation and TNF-α stimulation, and effects of a phosphodiesterase inhibitor. Life Sci. 80, 1721–1728 (2007).
McCarthy, E. T. et al. TNF-α increases albumin permeability of isolated rat glomeruli through the generation of superoxide. J. Am. Soc. Nephrol. 9, 433–438 (1998).
DiPetrillo, K., Coutermarsh, B. & Gesek, F. A. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am. J. Physiol. Renal Physiol. 284, F113–F121 (2003).
DiPetrillo, K. & Gesek, F. A. Pentoxifylline ameliorates renal tumor necrosis factor expression, sodium retention, and renal hypertrophy in diabetic rats. Am. J. Nephrol. 24, 352–359 (2004).
Schreiner, G. F. & Kohan, D. E. Regulation of renal transport processes and hemodynamics by macrophages and lymphocytes. Am. J. Physiol. 258, F761–F767 (1990).
Kalantarinia, K., Awas, A. S. & Siragy, H. M. Urinary and renal interstitial concentrations of TNF-α increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 64, 1208–1213 (2003).
Navarro, J. F., Mora, C., Macía, M. & García, J. Inflammatory parameters are independently associated with urinary albumin excretion in type 2 diabetes mellitus. Am. J. Kidney Dis. 42, 53–61 (2003).
Navarro, J. F., Mora, C., Muros, M. & García, J. Urinary tumor necrosis factor-α excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Nephrol. Dial. Transplant. 21, 3428–3434 (2006).
Niewczas, M. A. et al. Serum concentrations of markers of TNFα and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin. J. Am. Soc. Nephrol. 4, 62–70 (2009).
Lin, J., Hu, F. B., Mantzoros, C. & Curhan, G. C. Lipid and inflammatory biomarkers and kidney function decline in type 2 diabetes. Diabetologia 53, 263–267 (2010).
Schneider, P. et al. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-κB. Immunity 7, 831–836 (1997).
Lorz, C. et al. The death ligand TRAIL in diabetic nephropathy. J. Am. Soc. Nephrol. 19, 904–914 (2008).
Sanchez-Niño, M. D. et al. The MIF receptor CD74 in diabetic podocyte injury. J. Am. Soc. Nephrol. 20, 353–362 (2009).
Shikata, K. & Makino, H. Role of macrophages in the pathogenesis of diabetic nephropathy. Contrib. Nephrol. 134, 46–54 (2001).
Tesch, G. H. Role of macrophages in complications of type 2 diabetes. Clin. Exp. Pharmacol. Physiol. 34, 1016–1019 (2007).
Chow, F., Ozols, E., Nikolic-Paterson, D. J., Atkins, R. C. & Tesch, G. H. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 65, 116–128 (2004).
Bending, J. J., Lobo-Yeo, A., Vergani, D. & Viberti, G. C. Proteinuria and activated T-lymphocytes in diabetic nephropathy. Diabetes 37, 507–511 (1988).
Salozhin, K. V., Nasonov, E. L. & Sura, W. The cellular immunity indices in diabetic nephropathy [Russian]. Ter. Arkh. 63, 55–58 (1991).
Fardon, N. J., Wilkinson, R. & Thomas, T. H. Abnormalities in primary granule exocytosis in neutrophils from type I diabetic patients with nephropathy. Clin. Sci. (Lond.) 102, 69–75 (2002).
Takahashi, T. et al. Increased spontaneous adherence of neutrophils from type 2 diabetic patients with overt proteinuria: possible role of the progression of diabetic nephropathy. Diabetes Care 23, 417–418 (2000).
Galkina, E. & Ley, K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J. Am. Soc. Nephrol. 17, 368–377 (2006).
Min, D. et al. Mesangial cell-derived factors alter monocyte activation and function through inflammatory pathways: possible pathogenic role in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 297, F1229–F1237 (2009).
Ruster, C. & Wolf, G. The role of chemokines and chemokine receptors in diabetic nephropathy. Front. Biosci. 13, 944–955 (2008).
Panzer, U., Steinmetz, O. M., Stahl, R. A. & Wolf, G. Kidney diseases and chemokines. Curr. Drug Targets 7, 65–80 (2006).
Chow, Y. et al. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia 50, 471–480 (2007).
Chow, Y. et al. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 69, 73–80 (2006).
Tarabra, E. et al. Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes. Diabetes 54, 2109–2118 (2009).
Wada, T. et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 58, 1492–1498 (2000).
Tashiro, K. et al. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J. Clin. Lab. Anal. 16, 1–4 (2002).
Morii, T. et al. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J. Diabetes Complications 17, 11–15 (2003).
Lee, E. Y. et al. Monocyte chemoattractant protein-1/CCR2 loop, inducible by TGF-β, increases podocyte motility and albumin permeability. Am. J. Physiol. Renal Physiol. 297, F85–F94 (2009).
Janiak, P. et al. Long-term blockade of angiotensin AT1 receptors increases survival of obese Zucker rats. Eur. J. Pharmacol. 534, 271–279 (2006).
Mizuno, M. et al. The effect of angiotensin II receptor blockade on an end-stage renal failure model of type 2 diabetes. J. Cardiovasc. Pharmacol. 48, 135–142 (2006).
Amann, B., Tinzmann, R. & Angelkort, B. ACE inhibitors improve diabetic nephropathy through suppression of renal MCP-1. Diabetes Care 26, 2421–2425 (2003).
Kitagawa, K. et al. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am. J. Pathol. 165, 237–246 (2004).
Wada, T. et al. Gene therapy via blockade of monocyte chemoattractant protein-1 for renal fibrosis. J. Am. Soc. Nephrol. 15, 940–948 (2004).
Umehara, H. et al. Fractalkine in vascular biology: from basic research to clinical disease. Arterioscler. Thromb. Vasc. Biol. 24, 34–40 (2004).
Kikuchi, Y. et al. Fractalkine and its receptor, CX3CR1, upregulation in streptozotocin-induced diabetic kidneys. Nephron Exp. Nephrol. 97, e17–e25 (2004).
Kikuchi, Y. et al. Advanced glycation end-product induces fractalkine gene upregulation in normal rat glomeruli. Nephrol. Dial. Transplant. 20, 2690–2696 (2005).
Donadelli, R. et al. Protein overload induces fractalkine upregulation in proximal tubular cells through nuclear factor κB- and p38 mitogen-activated protein kinase-dependent pathways. J. Am. Soc. Nephrol. 14, 2436–2446 (2003).
Segerer, S. et al. Expression of the fractalkine receptor (CX3CR1) in human kidney diseases. Kidney Int. 62, 488–495 (2002).
Appay, V. & Rowland-Jones, S. L. RANTES: a versatile and controversial chemokine. Trends Immunol. 22, 83–87 (2001).
Herder, C. et al. Systemic immune mediators and lifestyle changes in the prevention of type 2 diabetes: results from the Finnish Diabetes Prevention Study. Diabetes 55, 2340–2346 (2006).
Mezzano, S. et al. NF-κB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol. Dial. Transplant. 19, 2505–2512 (2004).
Furuichi, K. et al. Distinct expression of CCR1 and CCR5 in glomerular and interstitial lesions of human glomerular diseases. Am. J. Nephrol. 20, 291–299 (2000).
Choi, J. S., Choi, Y. J., Park, S. H., Kang, J. S. & Kang, Y. H. Flavones mitigate tumor necrosis factor-α-induced adhesion molecule upregulation in cultured human endothelial cells: role of nuclear factor-κB. J. Nutr. 134, 1013–1019 (2004).
Sumagin, R. & Sarelius, I. H. TNF-α activation of arterioles and venules alters distribution and levels of ICAM-1 and affects leukocyte-endothelial cell interactions. Am. J. Physiol. Heart Circ. Physiol. 291, H2116–H2125 (2006).
Sucosky, P., Balachandran, K., Elhammali, A., Jo, H. & Yoganathan, A. P. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-β1-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 29, 254–260 (2009).
Okada, S. et al. Intercellular adhesion molecule-1 deficient mice are resistant against renal injury after induction of diabetes. Diabetes 52, 2586–2593 (2003).
Coimbra, T. M. et al. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 57, 167–182 (2000).
Lin, J. et al. Inflammation and progressive nephropathy in type 1 diabetes in the Diabetes Control and Complications Trial. Diabetes Care 31, 2338–2343 (2008).
Ina, K. et al. Vascular cell adhesion molecule-1 expression in the renal interstitium of diabetic KKAy mice. Diabetes Res. Clin. Pract. 44, 1–8 (1999).
Rubio-Guerra, A. F., Vargas-Robles, H., Lozano, J. J. & Escalante-Acosta, B. A. Correlation between circulating adhesion molecule levels and albuminuria in type-2 diabetic hypertensive patients. Kidney Blood Press. Res. 32, 106–109 (2009).
Stehouwer, C. D. et al. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes 51, 1157–1165 (2002).
Nasdala, I. et al. A transmembrane tight junction protein selectively expressed on endothelial cells and platelets. J. Biol. Chem. 277, 16294–16303 (2002).
Hara, T., Ishida, T., Cangara, H. M. & Hirata, K. Endothelial cell-selective adhesion molecule regulates albuminuria in diabetic nephropathy. Microvasc. Res. 77, 348–355 (2009).
Narumi, S., Onozato, M. L., Tojo, A., Sakamoto, S. & Tamatani, T. Tissue-specific induction of E-selectin in glomeruli is augmented following diabetes mellitus. Nephron 89, 161–171 (2001).
Hirata, K. et al. Increased expression of selectins in kidneys of patients with diabetic nephropathy. Diabetologia 41, 185–192 (1998).
Soedamah-Muthu, S. S. et al. Soluble vascular cell adhesion molecule-1 and soluble E-selectin are associated with micro- and macrovascular complications in type 1 diabetic patients. J. Diabetes Complications 20, 188–195 (2006).
Lopes-Virella, M. F. et al. Risk factors related to inflammation and endothelial dysfunction in the DCCT/EDIC cohort and their relationship with nephropathy and macrovascular complications. Diabetes Care 31, 2006–2012 (2008).
Dandapani, S. V. et al. α-Actinin-4 is required for normal podocyte adhesion. J. Biol. Chem. 282, 467–477 (2007).
Kimura, M. et al. Expression of alpha-actinin-4 in human diabetic nephropathy. Intern. Med. 47, 1099–1106 (2008).
Pieper, G. M. & Riaz-ul-Haq. Activation of nuclear factor-κ-B in cultured endothelial cells by increased glucose concentration: prevention by calphostin C. J. Cardiovasc. Pharmacol. 30, 528–532 (1997).
Yerneni, K. K., Bai, W., Khan, B. V., Medford, R. M. & Natarajan, R. Hyperglycemia-induced activation of nuclear transcription factor κB in vascular smooth muscle cells. Diabetes 48, 855–864 (1999).
Gruden, G. et al. Mechanical stretch induces monocyte chemoattractant activity via an NF-kappaB-dependent monocyte chemoattractant protein-1-mediated pathway in human mesangial cells: inhibition by rosiglitazone. J. Am. Soc. Nephrol. 16, 688–696 (2005).
Chuang, L.-Y. & Guh, J.-Y. Extracellular signals and intracellular pathways in diabetic nephropathy. Nephrology 6, 165–172 (2001).
Iwamoto, M., Mizuiri, S., Arita, M. & Hemmi, H. Nuclear factor-κB activation in diabetic rat kidney: evidence for involvement of P-selectin in diabetic nephropathy. Tohoku J. Exp. Med. 206, 163–171 (2005).
Guijarro, C. & Egido, J. Transcription factor-κB (NF-κB) and renal disease. Kidney Int. 59, 415–424 (2001).
Han, S. Y. et al. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J. Am. Soc. Nephrol. 17, 1362–1372 (2006).
Lee, F. T. et al. Interactions between angiotensin II and NF-κB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J. Am. Soc. Nephrol. 15, 2139–2151 (2004).
Schmid, H. et al. Modular activation of nuclear factor-κB transcriptional programs in human diabetic nephropathy. Diabetes 55, 2993–3003 (2006).
Hostetter, T. H. Prevention of end-stage renal disease due to type 2 diabetes. N. Engl. J. Med. 345, 910–912 (2001).
Williams, M. E. & Tuttle, K. R. The next generation of diabetic nephropathy therapies: an update. Adv. Chronic Kidney Dis. 12, 212–222 (2005).
Ohga, S. et al. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-κB activation. Am. J. Physiol. Renal Physiol. 292, F1141–F1150 (2007).
Ko, G. J. et al. Pioglitazone attenuates diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. Nephrol. Dial. Transplant. 23, 2750–2760 (2008).
Utimura, R. et al. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney Int. 63, 209–216 (2003).
Zhang, Y. et al. Effects of mycophenolate mofetil, valsartan and their combined therapy on preventing podocyte loss in early stage of diabetic nephropathy in rats. Chin. Med. J. (Engl.) 120, 988–995 (2007).
Rodríguez-Iturbe, B., Quiroz, Y., Shahkarami, A., Li., Z. & Vaziri, N. D. Mycophenolate mofetil ameliorates nephropathy in the obese Zucker rat. Kidney Int. 68, 1041–1047 (2005).
Moriwaki, Y. et al. Effect of TNF-α inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol. 44, 215–218 (2007).
Han, J., Thompson, P. & Beutler, D. Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J. Exp. Med. 172, 391–394 (1990).
Doherty, G. M., Jensen, J. C., Alexander, H. R., Buresh, C. M. & Norton, J. A. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery 110, 192–198 (1991).
Voisin, L. et al. Cytokine modulation by PX differently affects specific acute phase proteins during sepsis in rats. Am. J. Physiol. 275, R1412–R1419 (1998).
Cooper, A., Mikhail, A., Lethbridge, M. W., Kemeny, D. M. & Macdougall, I. C. Pentoxifylline improves hemoglobin levels in patients with erythropoietin-resistant anemia in renal failure. J. Am. Soc. Nephrol. 15, 1877–1882 (2004).
Bolick, D. T., Hatley, M. E., Srinivasan, S., Hedrick, C. C. & Nadler, J. L. Lisofylline, a novel antiinflammatory compound, protects mesangial cells from hyperglycemia- and angiotensin II-mediated extracellular matrix deposition. Endocrinology 144, 5227–5231 (2003).
Dávila-Esqueda, M. E., Vertiz-Hernández, A. A. & Martínez-Morales, F. Comparative analysis of the renoprotective effects of pentoxifylline and vitamin E on streptozotocin-induced diabetes mellitus. Ren. Fail. 27, 115–122 (2005).
Blagosklonnaia, IaV., Marnedov, R., Kozlov, V. V., Emanuél, V. L. & Kudriashova, M. I. Effect of trental on indices of kidney function in diabetes mellitus [Russian]. Probl. Endokrinol. (Mosk.) 28, 3–8 (1982).
Solerte, S. B. et al. Pentoxifylline, albumin excretion rate and proteinuria in type I and type II diabetic patients with microproteinuria. Results of a short-term randomized study. Acta Diabetol. Lat. 23, 171–177 (1986).
Tripathy, K., Praskash, J., Appaiha, D. & Srivastava, P. K. Pentoxifylline in management of proteinuria in diabetic nephropathy. Nephron 64, 641–642 (1993).
Guerrero-Romero, F. et al. Pentoxifylline reduces proteinuria in insulin-dependent and non insulin-dependent diabetic patients. Clin. Nephrol. 43, 116–121 (1995).
Gorson, D. M. Reduction of macroalbuminuria with pentoxifylline in diabetic nephropathy. Report of three cases. Diabetes Care 21, 2190–2191 (1998).
Navarro, J. F. & Mora, C. Antiproteinuric effect of pentoxifylline in patients with diabetic nephropathy. Diabetes Care 22, 1006–1008 (1999).
Navarro, J. F. et al. Urinary protein excretion and serum tumor necrosis factor in diabetic patients with advanced renal failure: effects of pentoxifylline administration. Am. J. Kidney Dis. 33, 458–463 (1999).
Navarro, J. F., Mora, C., Muros, M., Maca, M. & Garca, J. Effects of pentoxifylline administration on urinary N-acetyl-β-glucosaminidase excretion in type 2 diabetic patients: a short-term, prospective, randomized study. Am. J. Kidney Dis. 42, 264–270 (2003).
Rodriguez-Morán, M. et al. Effects of pentoxifylline on the urinary protein excretion profile of type 2 diabetic patients with microproteinuria: a double-blind, placebo-controlled randomized trial. Clin. Nephrol. 66, 3–10 (2006).
Leyva-Jiménez, R. et al. Effect of pentoxifylline on the evolution of diabetic nephropathy [Spanish]. Med. Clin. (Barc.) 132, 772–778 (2009).
Navarro, J. F., Mora, C., Muros, M. & García, J. Additive antiproteinuric effect of pentoxifylline in patients with type 2 diabetes under angiotensin II receptor blockade: a short-term, randomized, controlled trial. J. Am. Soc. Nephrol. 16, 2119–2126 (2005).
Roozbeh, J. et al. Captopril and combination therapy of captopril and pentoxifylline in reducing proteinuria in diabetic nephropathy. Ren. Fail. 32, 172–178 (2010).
McCormick, B. B. et al. The effect of pentoxifylline on proteinuria in diabetic kidney disease: a meta-analysis. Am. J. Kidney Dis. 52, 454–463 (2008).
Navarro-González, J. F. et al. Pentoxifylline for renoprotection in diabetic nephropathy: the PREDIAN study. Rationale and basal results. J. Diabetes Complications doi:10.1016/j.jdiacomp.2010.09.003.
Author information
Authors and Affiliations
Contributions
J. F. Navarro-González and C. Mora-Fernández contributed equally to all aspects of the article. M. Muros de Fuentes and J. García-Pérez researched data for the article, made substantial contributions to discussion of content as well as reviewing and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Navarro-González, J., Mora-Fernández, C., de Fuentes, M. et al. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7, 327–340 (2011). https://doi.org/10.1038/nrneph.2011.51
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2011.51
This article is cited by
-
Progress and application of adipose-derived stem cells in the treatment of diabetes and its complications
Stem Cell Research & Therapy (2024)
-
Effects of conditioned media derived from human Wharton’s jelly mesenchymal stem cells on diabetic nephropathy and hepatopathy via modulating TGF-β and apelin signaling pathways in male rats
BMC Endocrine Disorders (2024)
-
Effect of human umbilical cord blood-mesenchymal stem cells on cisplatin-induced nephrotoxicity in rats
Molecular Biology Reports (2024)
-
Effects of antioxidants on diabetic kidney diseases: mechanistic interpretations and clinical assessment
Chinese Medicine (2023)
-
Alpha‐kinase1 promotes tubular injury and interstitial inflammation in diabetic nephropathy by canonical pyroptosis pathway
Biological Research (2023)