Abstract
Injury to the podocyte results in proteinuria and often leads to progressive kidney disease. As podocytes have limited ability to repair and/or regenerate, the extent of podocyte injury is a major prognostic determinant in diabetic nephropathy and other common causes of end-stage renal disease. Therapies aimed at preventing or limiting podocyte injury and/or at promoting podocyte repair or regeneration therefore have major potential clinical and economic benefits. Many current therapies—including glucocorticosteroids and calcineurin antagonists—have potent effects on podocytes. The nonspecific natures of these agents lead to undesirable systemic adverse effects: an agent with a more specific focus on podocytes would cause less treatment-associated morbidity. Recent years have seen dramatic advances in our understanding of podocyte biology and in particular regulation of its actin cytoskeleton, the major determinant of the complex architecture on which these cells depend for their function. This advance has allowed the identification of potential therapeutic targets and the next few years should see the development and testing of specific therapies aimed at the podocyte. Thus we are about to move from a situation where some of our 'blunderbuss' older therapies fortuitously happened to have beneficial effects on podocytes to a new era where advances in biological knowledge about a key cell type in the kidney will allow targeted drug design. As well as being intellectually more satisfying, every reason exists to believe that patients of the future will benefit and that the scourge of progressive kidney disease will be more effectively tackled.
Key Points
-
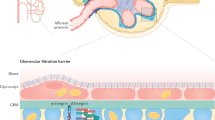
Proteinuria results from dysfunction of the glomerular capillary wall, the best studied component of which is the podocyte
-
Podocytes have limited ability to repair and/or regenerate
-
Many drugs currently used in the treatment of proteinuric disease were originally employed because of their immunotherapeutic and/or anti-inflammatory actions but it is not certain whether these actions explain their efficacy in renal disease
-
It is increasingly apparent that many currently used drugs have direct effects on podocytes
-
Rapid advances in our understanding of podocyte biology are leading to the identification of rational novel therapeutic targets
-
Future design of drugs for the treatment of proteinuric diseases should focus on podocyte repair and/or regeneration
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Patrakka, J. & Tryggvason, K. New insights into the role of podocytes in proteinuria. Nat. Rev. Nephrol. 5, 463–468 (2009).
Mathieson, P. W. Update on the podocyte. Curr. Opin. Nephrol. Hypertens. 18, 206–211 (2009).
Hlatky, M. A. Is renal biopsy necessary in adults with nephrotic syndrome. Lancet 2, 1264–1268 (1982).
Ransom, R. F., Lam, N. G., Hallett, M. A., Atkinson, S. J. & Smoyer, W. E. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int. 68, 2473–2483 (2005).
Xing, C. Y. et al. Direct effects of dexamethasone on human podocytes. Kidney Int. 70, 1038–1045 (2006).
Wada, T., Pippin, J. W., Marshall, C. B., Griffin, S. V. & Shankland, S. J. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J. Am. Soc. Nephrol. 16, 2615–2625 (2005).
Fuji, Y. et al. The effect of dexamethasone on defective nephrin transport caused by ER stress: a potential mechanism for the therapeutic action of glucocorticoids in the acquired glomerular diseases. Kidney Int. 69, 1350–1359 (2006).
Guess, A. et al. Dose- and time-dependent glucocorticoid receptor signaling in podocytes. Am. J. Physiol. Renal Physiol. 299, F845–F853 (2010).
Ohashi, T., Uchida, K., Uchida, S., Sasaki, S. & Nitta, K. Dexamethasone increases the phosphorylation of nephrin in cultured podocytes. Clin. Exp. Nephrol. doi:10.1007/s10157-011-0479-0.
Uchida, K. et al. Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int. 73, 926–932 (2008).
Hussain, S. et al. Nephrin deficiency activates NF-kappaB and promotes glomerular injury. J. Am. Soc. Nephrol. 20, 1733–1743 (2009).
Mudge, S. J. et al. Corticosteroids worsen proteinuria and increase intraglomerular signaling by NF-kB in a model of membranous glomerulonephritis. Nephron Exp. Nephrol. 116, e23–e31 (2010).
Lindskog, A. et al. Melanocortin 1 receptor agonists reduce proteinuria. J. Am. Soc. Nephrol. 21, 1290–1298 (2010).
Faul, C. et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 14, 931–938 (2008).
Bensman, A. & Niaudet, P. Non-immunologic mechanisms of calcineurin inhibitors, explain its antiproteinuric effects in genetic glomerulopathies. Pediatric Nephrol. 25, 1197–1199 (2010).
Torras, J. et al. Rapamycin has dual opposing effects on proteinuric experimental nephropathies: is it a matter of podocyte damage? Nephrol. Dial. Transplant. 24, 3632–3640 (2009).
Stallone, G. et al. Sirolimus and proteinuria in renal transplant patients: evidence for a dose-dependent effect on slit diaphragm proteins. Transplantation 91, 997–1004 (2011).
Inoki, K. et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 121, 2181–2196 (2011).
Gödel, M. et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest. 121, 2197–2209 (2011).
Mathieson, P. W. Clinical Implications of basic research: proteinuria and immunity—an over-stated relationship? N. Engl. J. Med. 359, 2492–2494 (2008).
Fornoni, A. et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci. Transl. Med. 3, 85ra46 (2011).
Szeto, C. C., Gillespie, K. M. & Mathieson, P. W. Levamisole induces interleukin-18 and shifts type 1/type 2 cytokine balance. Immunology 100, 217–224 (2000).
Harris, J. J., Welsh, G. I., Mathieson, P. W. & Saleem, M. A. FSGS plasma initiates specific signalling pathways in podocytes—evidence for an imbalance of circulating proteases [abstract TH-PO908]. Presented at The American Society of Nephrology Renal Week 2009.
Winn, M. P. et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308, 1801–1804 (2005).
Puri, A., McGoon, M. D. & Kushwaha, S. S. Pulmonary arterial hypertension: current therapeutic strategies. Nat. Clin. Pract. Cardiovasc. Med. 4, 319–329 (2007).
Komers, R. Rho kinase inhibition in diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 20, 77–83 (2011).
Macconi, D. et al. Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am. J. Pathol. 174, 797–807 (2009).
Fukuda, A., Fujimoto, S., Iwatsubo, S., Kawachi, H. & Kitamura, K. Effects of mineralocorticoid and angiotensin II receptor blockers on proteinuria and glomerular podocyte protein expression in a model of minimal change nephrotic syndrome. Nephrology (Carlton) 15, 321–326 (2010).
Nishiyama, A. et al. Mineralocorticoid receptor blockade enhances the antiproteinuric effect of an angiotensin II blocker through inhibiting podocyte injury in type 2 diabetic rats. J. Pharmacol. Exp. Ther. 332, 1072–1080 (2010).
Lin, S. et al. Spironolactone ameliorates podocytic adhesive capacity via restoring integrin alpha 3 expression in streptozotocin-induced diabetic rats. J. Renin Angiotensin Aldosterone Syst. 11, 149–157 (2010).
Okada, T. et al. Tolvaptan, a selective oral vasopressin V2 receptor antagonist, ameliorates podocyte injury in puromycin aminonucleoside nephrotic rats. Clin. Exp. Nephrol. 13, 438–446 (2009).
Sakurai, N. et al. Fluvastatin prevents podocyte injury in a murine model of HIV-associated nephropathy. Nephrol. Dial. Transplant. 24, 2378–2383 (2009).
Wei, P. et al. Simvastatin reverses podocyte injury but not mesangial expansion in early stage type 2 diabetes mellitus. Ren. Fail. 31, 503–513 (2009).
Zhou, Y. et al. Peroxisome proliferator-activated receptor-α is renoprotective in doxorubicin-induced glomerular injury. Kidney Int. 79, 1302–1311 (2011).
Eto, N. et al. Podocyte protection by darbepoetin: preservation of the cytoskeleton and nephrin expression. Kidney Int. 72, 455–463 (2007).
Schiffer, M. et al. Erythropoietin prevents diabetes-induced podocyte damage. Kidney Blood Press. Res. 31, 411–415 (2008).
Ruester, C., Franke, S., Bondeva, T. & Wolf, G. Erythropoietin protects podocytes from damage by advanced glycation end-products. Nephron Exp. Nephrol. 117, e21–e30 (2011).
Takeuchi, S. et al. The immunosuppressive drug mizoribine directly prevents podocyte injury in puromycin aminonucleoside nephrosis. Nephron Exp. Nephrol. 116, e3–e10 (2010).
Chen, Z. H. et al. Triptolide reduces proteinuria in experimental membranous nephropathy and protects against C5b-9-induced podocyte injury in vitro. Kidney Int. 77, 974–988 (2010).
Gao, Q. et al. Treatment of db/db diabetic mice with triptolide: a novel therapy for diabetic nephropathy. Nephrol. Dial. Transplant. 25, 3539–3547 (2010).
Dai, C., Saleem, M. A., Holzman, L. B., Mathieson, P. & Liu, Y. Hepatocyte growth factor signaling ameliorates podocyte injury and proteinuria. Kidney Int. 77, 962–973 (2010).
Kato, T., Mizuno, S. & Nakamura, T. Preservations of nephrin and synaptopodin by recombinant hepatocyte growth factor in podocytes for the attenuations of foot process injury and albuminuria in nephritic mice. Nephrology (Carlton) 16, 310–318 (2011).
Satchell, S. C. et al. Interferon-beta reduces proteinuria in experimental glomerulonephritis. J. Am. Soc. Nephrol. 18, 2875–2884 (2007).
Kang, Y. S. et al. Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int. 78, 363–373 (2010).
Ma, H. et al. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J. Am. Soc. Nephrol. 21, 1145–1156 (2010).
Agarwal, R. Are vitamin D receptor agonists like angiotensin-converting enzyme inhibitors without side effects? Kidney Int. 77, 943–945 (2010).
Zou, M. S. et al. 1, 25-dihydroxyvitamin D3 decreases adriamycin-induced podocyte apoptosis and loss. Int. J. Med. Sci. 7, 290–299 (2010).
Kwak, S. J. et al. Local kallikrein-kinin system is involved in podocyte apoptosis under diabetic conditions. Apoptosis 16, 478–490 (2011).
Barutta, F. et al. Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes 59, 1046–1054 (2010).
Ahn, S. H. & Susztak, K. Getting a notch closer to understanding diabetic kidney disease. Diabetes 59, 1865–1867 (2010).
Welsh, G. I. et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 12, 329–340 (2010).
Piwkowska, A. et al. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem. Biophys. Res. Commun. 393, 268–273 (2010).
Liu, H. F. et al. Thiazolidinedione attenuate proteinuria and glomerulosclerosis in Adriamycin-induced nephropathy rats via slit diaphragm protection. Nephrology (Carlton) 15, 75–83 (2010).
Mironidou-Tzouveleki, M., Tsartsalis, S. & Tomos, C. Vascular endothelial growth factor (VEGF) in the pathogenesis of diabetic nephropathy of type 1 diabetes mellitus. Curr. Drug Targets. 12, 107–114 (2011).
Saito, D. et al. Amelioration of renal alterations in obese type 2 diabetic mice by vasohibin-1, a negative feedback regulator of angiogenesis. Am. J. Physiol. Renal Physiol. 300, F873–F886 (2011).
Appel, D. et al. Recruitment of podocytes from glomerular parietal epithelial cells. J. Am. Soc. Nephrol. 20, 333–343 (2009).
Roufosse, C. & Cook, H. T. Stem cells and renal regeneration. Nephron Exp. Nephrol. 109, e39–e45 (2008).
Becker, J. U., Hoerning, A., Schmid, K. W. & Hoyer, P. F. Immigrating progenitor cells contribute to human podocyte turnover. Kidney Int. 72, 1468–1473 (2007).
Lasagni, L. & Romagnani, P. Glomerular epithelial stem cells: the good, the bad, and the ugly. J. Am. Soc. Nephrol. 21, 1612–1619 (2010).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Mathieson, P. The podocyte as a target for therapies—new and old. Nat Rev Nephrol 8, 52–56 (2012). https://doi.org/10.1038/nrneph.2011.171
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2011.171
This article is cited by
-
Glomerular injury after trauma, burn, and sepsis
Journal of Nephrology (2023)
-
Emodin Ameliorates High Glucose-Induced Podocyte Apoptosis via Regulating AMPK/mTOR-Mediated Autophagy Signaling Pathway
Chinese Journal of Integrative Medicine (2023)
-
Asparaginyl endopeptidase protects against podocyte injury in diabetic nephropathy through cleaving cofilin-1
Cell Death & Disease (2022)
-
METTL14 aggravates podocyte injury and glomerulopathy progression through N6-methyladenosine-dependent downregulating of Sirt1
Cell Death & Disease (2021)
-
Astragaloside IV inhibits palmitic acid-induced apoptosis through regulation of calcium homeostasis in mice podocytes
Molecular Biology Reports (2021)