Abstract
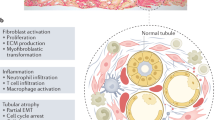
Renal fibrosis, particularly tubulointerstitial fibrosis, is the common final outcome of almost all progressive chronic kidney diseases. Renal fibrosis is also a reliable predictor of prognosis and a major determinant of renal insufficiency. Irrespective of the initial causes, renal fibrogenesis is a dynamic and converging process that consists of four overlapping phases: priming, activation, execution and progression. Nonresolving inflammation after a sustained injury sets up the fibrogenic stage (priming) and triggers the activation and expansion of matrix-producing cells from multiple sources through diverse mechanisms, including activation of interstitial fibroblasts and pericytes, phenotypic conversion of tubular epithelial and endothelial cells and recruitment of circulating fibrocytes. Upon activation, matrix-producing cells assemble a multicomponent, integrin-associated protein complex that integrates input from various fibrogenic signals and orchestrates the production of matrix components and their extracellular assembly. Multiple cellular and molecular events, such as tubular atrophy, microvascular rarefaction and tissue hypoxia, promote scar formation and ensure a vicious progression to end-stage kidney failure. This Review outlines our current understanding of the cellular and molecular mechanisms of renal fibrosis, which could offer novel insights into the development of new therapeutic strategies.
Key Points
-
Despite having various initial causes, renal fibrogenesis is a converging and highly dynamic process, which consists of four overlapping phases: priming, activation, execution and progression
-
After a sustained injury, nonresolving inflammation sets up a fibrogenic stage (priming) and triggers the activation and expansion of matrix-producing fibroblasts from multiple sources through a range of mechanisms
-
Upon activation, the matrix-producing cells build a multicomponent, integrin-associated, ternary protein complex, which integrates various fibrogenic signals and orchestrates the production of extracellular matrix and its assembly
-
Many cellular and molecular events, such as tubular atrophy, vascular rarefaction and hypoxia, promote the progressive loss of kidney function and determine the outcome of renal fibrosis
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Coresh, J. et al. Prevalence of chronic kidney disease in the United States. JAMA 298, 2038–2047 (2007).
United States Renal Data System. Annual Data Report 2009 [online], (2010).
Sharma, S. K. et al. Burden of CKD, proteinuria, and cardiovascular risk among Chinese, Mongolian, and Nepalese participants in the International Society of Nephrology screening programs. Am. J. Kidney Dis. 56, 915–927 (2010).
Zhang, L. et al. Prevalence and factors associated with CKD: a population study from Beijing. Am. J. Kidney Dis. 51, 373–384 (2008).
Liu, Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 69, 213–217 (2006).
Wynn, T. A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214, 199–210 (2008).
Zeisberg, M. & Neilson, E. G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 21, 1819–1834 (2010).
Boor, P., Ostendorf, T. & Floege, J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat. Rev. Nephrol. 6, 643–656 (2010).
Li, Y. et al. Epithelial-to-mesenchymal transition is a potential pathway leading to podocyte dysfunction and proteinuria. Am. J. Pathol. 172, 299–308 (2008).
Yamaguchi, Y. et al. Epithelial-mesenchymal transition as an explanation for podocyte depletion in diabetic nephropathy. Am. J. Kidney Dis. 54, 653–664 (2009).
Kang, Y. S. et al. Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int. 78, 363–373 (2010).
Eddy, A. A. Molecular basis of renal fibrosis. Pediatr. Nephrol. 15, 290–301 (2000).
Chung, A. C. & Lan, H. Y. Chemokines in renal injury. J. Am. Soc. Nephrol. 22, 802–809 (2011).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Nathan, C. & Ding, A. Nonresolving inflammation. Cell 140, 871–882 (2010).
Vielhauer, V., Kulkarni, O., Reichel, C. A. & Anders, H. J. Targeting the recruitment of monocytes and macrophages in renal disease. Semin. Nephrol. 30, 318–333 (2010).
Vernon, M. A., Mylonas, K. J. & Hughes, J. Macrophages and renal fibrosis. Semin. Nephrol. 30, 302–317 (2010).
Duffield, J. S. Macrophages and immunologic inflammation of the kidney. Semin. Nephrol. 30, 234–254 (2010).
Ricardo, S. D., van Goor, H. & Eddy, A. A. Macrophage diversity in renal injury and repair. J. Clin. Invest. 118, 3522–3530 (2008).
Wang, Y. & Harris, D. C. Macrophages in renal disease. J. Am. Soc. Nephrol. 22, 21–27 (2011).
Grande, M. T., Pérez-Barriocanal, F. & López-Novoa, J. M. Role of inflammation in tubulo-interstitial damage associated to obstructive nephropathy. J. Inflamm. 7, 19 (2010).
Tapmeier, T. T. et al. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 78, 351–362 (2010).
Lin, S. L., Castaño, A. P., Nowlin, B. T., Lupher, M. L. Jr & Duffield, J. S. Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J. Immunol. 183, 6733–6743 (2009).
Henderson, N. C. et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am. J. Pathol. 172, 288–298 (2008).
Ko, G. J., Boo, C. S., Jo, S. K., Cho, W. Y. & Kim, H. K. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol. Dial. Transplant. 23, 842–852 (2008).
Wang, Y. et al. By homing to the kidney, activated macrophages potently exacerbate renal injury. Am. J. Pathol. 172, 1491–1499 (2008).
Kaissling, B. & Le Hir, M. The renal cortical interstitium: morphological and functional aspects. Histochem. Cell Biol. 130, 247–262 (2008).
John, R. & Nelson, P. J. Dendritic cells in the kidney. J. Am. Soc. Nephrol. 18, 2628–2635 (2007).
Teteris, S. A., Engel, D. R. & Kurts, C. Homeostatic and pathogenic role of renal dendritic cells. Kidney Int. 80, 139–145 (2011).
Macconi, D. et al. Proteasomal processing of albumin by renal dendritic cells generates antigenic peptides. J. Am. Soc. Nephrol. 20, 123–130 (2009).
Heymann, F. et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J. Clin. Invest. 119, 1286–1297 (2009).
Hochheiser, K. et al. Kidney dendritic cells become pathogenic during crescentic glomerulonephritis with proteinuria. J. Am. Soc. Nephrol. 22, 306–316 (2011).
Timoshanko, J. R., Kitching, A. R., Semple, T. J., Tipping, P. G. & Holdsworth, S. R. A pathogenetic role for mast cells in experimental crescentic glomerulonephritis. J. Am. Soc. Nephrol. 17, 150–159 (2006).
Holdsworth, S. R. & Summers, S. A. Role of mast cells in progressive renal diseases. J. Am. Soc. Nephrol. 19, 2254–2261 (2008).
Kanamaru, Y. et al. Mast cell-mediated remodeling and fibrinolytic activity protect against fatal glomerulonephritis. J. Immunol. 176, 5607–5615 (2006).
Anders, H. J. et al. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J. Clin. Invest. 109, 251–259 (2002).
Sayyed, S. G. et al. An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int. 80, 68–78 (2011).
Tan, X., Wen, X. & Liu, Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-κB signaling. J. Am. Soc. Nephrol. 19, 1741–1752 (2008).
Wen, X., Li, Y. & Liu, Y. Opposite action of peroxisome proliferator-activated receptor-γ in regulating renal inflammation: functional switch by its ligand. J. Biol. Chem. 285, 29981–29988 (2010).
Kawai, T. et al. PPAR-γ agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-β. Lab. Invest. 89, 47–58 (2009).
Khan, S. B. et al. Antibody blockade of TNF-α reduces inflammation and scarring in experimental crescentic glomerulonephritis. Kidney Int. 67, 1812–1820 (2005).
Giannopoulou, M. et al. Hepatocyte growth factor exerts its anti-inflammatory action by disrupting nuclear factor-κB signaling. Am. J. Pathol. 173, 30–41 (2008).
Gong, R., Rifai, A. & Dworkin, L. D. Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: targeting the inflamed vascular endothelium. J. Am. Soc. Nephrol. 17, 2464–2473 (2006).
Jones, L. K. et al. IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrol. Dial. Transplant. 24, 3024–3032 (2009).
Yu, C., Gong, R., Rifai, A., Tolbert, E. M. & Dworkin, L. D. Long-term, high-dosage candesartan suppresses inflammation and injury in chronic kidney disease: nonhemodynamic renal protection. J. Am. Soc. Nephrol. 18, 750–759 (2007).
Pate, M., Damarla, V., Chi, D. S., Negi, S. & Krishnaswamy, G. Endothelial cell biology: role in the inflammatory response. Adv. Clin. Chem. 52, 109–130 (2010).
López-Novoa, J. M. & Nieto, M. A. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 1, 303–314 (2009).
Nightingale, J. et al. Oncostatin M, a cytokine released by activated mononuclear cells, induces epithelial cell-myofibroblast transdifferentiation via Jak/Stat pathway activation. J. Am. Soc. Nephrol. 15, 21–32 (2004).
Li, Q. et al. Monocytes induce proximal tubular epithelial mesenchymal transition through NF-κB dependent upregulation of ICAM-1. J. Cell. Biochem. 112, 1585–1592 (2011).
Wu, Y. et al. Stabilization of snail by NF-κB is required for inflammation-induced cell migration and invasion. Cancer Cell 15, 416–428 (2009).
Boutet, A. et al. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J. 25, 5603–5613 (2006).
Rowe, R. G. et al. Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J. Cell Biol. 184, 399–408 (2009).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Inoue, T. et al. Fibroblast expression of an IκB dominant-negative transgene attenuates renal fibrosis. J. Am. Soc. Nephrol. 21, 2047–2052 (2010).
Li, Y., Yang, J., Luo, J. H., Dedhar, S. & Liu, Y. Tubular epithelial cell dedifferentiation is driven by the helix-loop-helix transcriptional inhibitor Id1. J. Am. Soc. Nephrol. 18, 449–460 (2007).
Yang, Y., Liou, H. C. & Sun, X. H. Id1 potentiates NF-κB activation upon T cell receptor signaling. J. Biol. Chem. 281, 34989–34996 (2006).
Lin, J. et al. Inhibitor of differentiation 1 contributes to head and neck squamous cell carcinoma survival via the NF-κB/survivin and phosphoinositide 3-kinase/Akt signaling pathways. Clin. Cancer Res. 16, 77–87 (2010).
Duffield, J. S. Macrophages in kidney repair and regeneration. J. Am. Soc. Nephrol. 22, 199–201 (2011).
Gandolfo, M. T. et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 76, 717–729 (2009).
Cao, Q. et al. IL-10/TGF-β-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J. Am. Soc. Nephrol. 21, 933–942 (2010).
Meran, S. & Steadman, R. Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol. 92, 158–167 (2011).
Grande, M. T. & López-Novoa, J. M. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat. Rev. Nephrol. 5, 319–328 (2009).
Schrimpf, C. & Duffield, J. S. Mechanisms of fibrosis: the role of the pericyte. Curr. Opin. Nephrol. Hypertens. 20, 297–305 (2011).
Barnes, J. L. & Gorin, Y. Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases. Kidney Int. 79, 944–956 (2011).
Hewitson, T. D. Renal tubulointerstitial fibrosis: common but never simple. Am. J. Physiol. Renal Physiol. 296, F1239–F1244 (2009).
Strutz, F. & Zeisberg, M. Renal fibroblasts and myofibroblasts in chronic kidney disease. J. Am. Soc. Nephrol. 17, 2992–2998 (2006).
Paliege, A. et al. Hypoxia-inducible factor-2α-expressing interstitial fibroblasts are the only renal cells that express erythropoietin under hypoxia-inducible factor stabilization. Kidney Int. 77, 312–318 (2010).
Boor, P. & Floege, J. Chronic kidney disease growth factors in renal fibrosis. Clin. Exp. Pharmacol. Physiol. 38, 391–400 (2011).
Floege, J., Eitner, F. & Alpers, C. E. A new look at platelet-derived growth factor in renal disease. J. Am. Soc. Nephrol. 19, 12–23 (2008).
Boye, K. & Maelandsmo, G. M. S100A4 and metastasis: a small actor playing many roles. Am. J. Pathol. 176, 528–535 (2010).
Grigorian, M., Ambartsumian, N. & Lukanidin, E. Metastasis-inducing S100A4 protein: implication in non-malignant human pathologies. Curr. Mol. Med. 8, 492–496 (2008).
Wynn, T. A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J. Clin. Invest. 117, 524–529 (2007).
Hinz, B. et al. The myofibroblast: one function, multiple origins. Am. J. Pathol. 170, 1807–1816 (2007).
Lin, S. L., Kisseleva, T., Brenner, D. A. & Duffield, J. S. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am. J. Pathol. 173, 1617–1627 (2008).
Takeji, M. et al. Smooth muscle α-actin deficiency in myofibroblasts leads to enhanced renal tissue fibrosis. J. Biol. Chem. 281, 40193–40200 (2006).
Zou, J. et al. Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch. 448, 485–492 (2006).
Phanish, M. K., Winn, S. K. & Dockrell, M. E. Connective tissue growth factor-(CTGF, CCN2)—a marker, mediator and therapeutic target for renal fibrosis. Nephron Exp. Nephrol. 114, e83–e92 (2010).
Böttinger, E. P. TGF-β in renal injury and disease. Semin. Nephrol. 27, 309–320 (2007).
Strutz, F. et al. Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int. 57, 1521–1538 (2000).
Ostendorf, T., Eitner, F. & Floege, J. The PDGF family in renal fibrosis. Pediatr. Nephrol. http://dx.doi.org/10.1007/s00467-011-1892-z.
Hu, K. et al. tPA protects renal interstitial fibroblasts and myofibroblasts from apoptosis. J. Am. Soc. Nephrol. 19, 503–514 (2008).
Hao, S., Shen, H., Hou, Y., Mars, W. M. & Liu, Y. tPA is a potent mitogen for renal interstitial fibroblasts: role of β1 integrin/focal adhesion kinase. Am. J. Pathol. 177, 1164–1175 (2010).
Lin, L. et al. tPA activates LDL receptor-related protein 1-mediated mitogenic signaling involving the p90RSK and GSK3β pathway. Am. J. Pathol. 177, 1687–1696 (2010).
Hu, K., Wu, C., Mars, W. M. & Liu, Y. Tissue-type plasminogen activator promotes murine myofibroblast activation through LDL receptor-related protein 1-mediated integrin signaling. J. Clin. Invest. 117, 3821–3832 (2007).
Hu, K. et al. Tissue-type plasminogen activator acts as a cytokine that triggers intracellular signal transduction and induces matrix metalloproteinase-9 gene expression. J. Biol. Chem. 281, 2120–2127 (2006).
Duffield, J. S. & Humphreys, B. D. Origin of new cells in the adult kidney: results from genetic labeling techniques. Kidney Int. 79, 494–501 (2011).
Humphreys, B. D. et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am. J. Pathol. 176, 85–97 (2010).
Kalluri, R. & Weinberg, R. A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009).
Acloque, H., Adams, M. S., Fishwick, K., Bronner-Fraser, M. & Nieto, M. A. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J. Clin. Invest. 119, 1438–1449 (2009).
Sleeman, J. P. & Thiery, J. P. SnapShot: the epithelial-mesenchymal transition. Cell 145, 162.e1 (2011).
Li, J., Qu, X. & Bertram, J. F. Endothelial-myofibroblast transition contributes to the early development of diabetic renal interstitial fibrosis in streptozotocin-induced diabetic mice. Am. J. Pathol. 175, 1380–1388 (2009).
Zeisberg, E. M., Potenta, S. E., Sugimoto, H., Zeisberg, M. & Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 19, 2282–2287 (2008).
Kriz, W., Kaissling, B. & Le Hir, M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J. Clin. Invest. 121, 468–474 (2011).
Zeisberg, M. & Duffield, J. S. Resolved: EMT produces fibroblasts in the kidney. J. Am. Soc. Nephrol. 21, 1247–1253 (2010).
Liu, Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 21, 212–222 (2010).
Li, J. & Bertram, J. F. Endothelial-myofibroblast transition, a new player in diabetic renal fibrosis. Nephrology 15, 507–512 (2010).
Burns, W. C. & Thomas, M. C. The molecular mediators of type 2 epithelial to mesenchymal transition (EMT) and their role in renal pathophysiology. Expert Rev. Mol. Med. 12, e17 (2010).
Grgic, I., Duffield, J. S. & Humphreys, B. D. The origin of interstitial myofibroblasts in chronic kidney disease. Pediatr. Nephrol. http://dx.doi.org/10.1007/s00467-011-1772-6.
Quaggin, S. E. & Kapus, A. Scar wars: mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int. 80, 41–50 (2011).
Yang, J. & Liu, Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am. J. Pathol. 159, 1465–1475 (2001).
Iwano, M. et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110, 341–350 (2002).
Li, L., Zepeda-Orozco, D., Black, R. & Lin, F. Autophagy is a component of epithelial cell fate in obstructive uropathy. Am. J. Pathol. 176, 1767–1778 (2010).
Togawa, H. et al. Epithelial-to-mesenchymal transition in cyst lining epithelial cells in an orthologous PCK rat model of autosomal-recessive polycystic kidney disease. Am. J. Physiol. Renal Physiol. 300, F511–F520 (2011).
Boonla, C. et al. Fibrosis and evidence for epithelial-mesenchymal transition in the kidneys of patients with staghorn calculi. BJU Int. 108, 1336–1345 (2011).
Yang, J. & Liu, Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J. Am. Soc. Nephrol. 13, 96–107 (2002).
Zeisberg, M. et al. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9, 964–968 (2003).
Hertig, A. et al. Early epithelial phenotypic changes predict graft fibrosis. J. Am. Soc. Nephrol. 19, 1584–1591 (2008).
Galichon, P. & Hertig, A. Epithelial to mesenchymal transition as a biomarker in renal fibrosis: are we ready for the bedside? Fibrogenesis Tissue Repair 4, 11 (2011).
He, W. et al. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 765–776 (2009).
Li, Y. et al. Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J. Am. Soc. Nephrol. 20, 1907–1918 (2009).
Yang, J. et al. Disruption of tissue-type plasminogen activator gene in mice reduces renal interstitial fibrosis in obstructive nephropathy. J. Clin. Invest. 110, 1525–1538 (2002).
Liu, Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J. Am. Soc. Nephrol. 15, 1–12 (2004).
Surendran, K., Schiavi, S. & Hruska, K. A. Wnt-dependent β-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J. Am. Soc. Nephrol. 16, 2373–2384 (2005).
Tan, X., Li, Y. & Liu, Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J. Am. Soc. Nephrol. 17, 3382–3393 (2006).
Zhou, Y. et al. HSP72 inhibits Smad3 activation and nuclear translocation in renal epithelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 21, 598–609 (2010).
Herzog, E. L. & Bucala, R. Fibrocytes in health and disease. Exp. Hematol. 38, 548–556 (2010).
Wada, T. et al. Involvement of bone-marrow-derived cells in kidney fibrosis. Clin. Exp. Nephrol. 15, 8–13 (2011).
Pilling, D., Fan, T., Huang, D., Kaul, B. & Gomer, R. H. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE 4, e7475 (2009).
Niedermeier, M. et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc. Natl Acad. Sci. USA 106, 17892–17897 (2009).
Shao, D. D., Suresh, R., Vakil, V., Gomer, R. H. & Pilling, D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J. Leukoc. Biol. 83, 1323–1333 (2008).
Sakai, N. et al. The renin-angiotensin system contributes to renal fibrosis through regulation of fibrocytes. J. Hypertens. 26, 780–790 (2008).
Roufosse, C. et al. Bone marrow-derived cells do not contribute significantly to collagen I synthesis in a murine model of renal fibrosis. J. Am. Soc. Nephrol. 17, 775–782 (2006).
Yang, J., Dai, C. & Liu, Y. Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am. J. Pathol. 163, 621–632 (2003).
Schnaper, H. W. et al. TGF-β signal transduction in chronic kidney disease. Front. Biosci. 14, 2448–2465 (2009).
Dai, C. & Liu, Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-β1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J. Am. Soc. Nephrol. 15, 1402–1412 (2004).
Liu, Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am. J. Physiol. Renal Physiol. 287, F7–F16 (2004).
Luo, D. D., Phillips, A. & Fraser, D. Bone morphogenetic protein-7 inhibits proximal tubular epithelial cell Smad3 signaling via increased SnoN expression. Am. J. Pathol. 176, 1139–1147 (2010).
Wang, B. et al. miR-200a prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes 60, 280–287 (2011).
Kato, M. et al. A microRNA circuit mediates transforming growth factor-β1 autoregulation in renal glomerular mesangial cells. Kidney Int. 80, 358–368 (2011).
Inui, M., Martello, G. & Piccolo, S. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol. 11, 252–263 (2010).
Chung, A. C., Huang, X. R., Meng, X. & Lan, H. Y. miR-192 mediates TGF-β/Smad3-driven renal fibrosis. J. Am. Soc. Nephrol. 21, 1317–1325 (2010).
Zhou, Q. et al. TGF-β-induced MiR-491-5p expression promotes Par-3 degradation in rat proximal tubular epithelial cells. J. Biol. Chem. 285, 40019–40027 (2010).
Eyden, B. Fibronexus junctions associated with in vivo human endothelium. Ultrastruct. Pathol. 33, 28–32 (2009).
Margadant, C. & Sonnenberg, A. Integrin-TGF-β crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11, 97–105 (2010).
Legate, K. R. & Fässler, R. Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J. Cell Sci. 122, 187–198 (2009).
Legate, K. R., Montañez, E., Kudlacek, O. & Fässler, R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 7, 20–31 (2006).
Maydan, M. et al. Integrin-linked kinase is a functional Mn2+-dependent protein kinase that regulates glycogen synthase kinase-3β (GSK-3β) phosphorylation. PLoS ONE 5, e12356 (2010).
Fukuda, K., Gupta, S., Chen, K., Wu, C. & Qin, J. The pseudoactive site of ILK is essential for its binding to α-parvin and localization to focal adhesions. Mol. Cell 36, 819–830 (2009).
He, W. et al. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/β-catenin signaling. J. Biol. Chem. 285, 24665–24675 (2010).
ten Berge, D. et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 3, 508–518 (2008).
Wu, C. PINCH, N(i)ck and the ILK: network wiring at cell-matrix adhesions. Trends Cell Biol. 15, 460–466 (2005).
Li, Y., Dai, C., Wu, C. & Liu, Y. PINCH-1 promotes tubular epithelial-to-mesenchymal transition by interacting with integrin-linked kinase. J. Am. Soc. Nephrol. 18, 2534–2543 (2007).
Guo, L. & Wu, C. Regulation of fibronectin matrix deposition and cell proliferation by the PINCH–ILK–CH–ILKBP complex. FASEB J. 16, 1298–1300 (2002).
Yeh, Y. C. et al. Transforming growth factor-β1 induces Smad3-dependent β1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am. J. Pathol. 177, 1743–1754 (2010).
Li, Y., Yang, J., Dai, C., Wu, C. & Liu, Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J. Clin. Invest. 112, 503–516 (2003).
Liu, X. C., Liu, B. C., Zhang, X. L., Li, M. X. & Zhang, J. D. Role of ERK1/2 and PI3-K in the regulation of CTGF-induced ILK expression in HK-2 cells. Clin. Chim. Acta 382, 89–94 (2007).
Han, S. Y. et al. High glucose and angiotensin II increase β1 integrin and integrin-linked kinase synthesis in cultured mouse podocytes. Cell Tissue Res. 323, 321–332 (2006).
Yang, F. et al. Essential role for Smad3 in angiotensin II-induced tubular epithelial-mesenchymal transition. J. Pathol. 221, 390–401 (2010).
Carvajal, G. et al. Angiotensin II activates the Smad pathway during epithelial mesenchymal transdifferentiation. Kidney Int. 74, 585–595 (2008).
Yang, F., Chung, A. C., Huang, X. R. & Lan, H. Y. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-β-dependent and -independent Smad pathways: the role of Smad3. Hypertension 54, 877–884 (2009).
Eddy, A. A. Progression in chronic kidney disease. Adv. Chronic Kidney Dis. 12, 353–365 (2005).
Bradshaw, A. D. The role of SPARC in extracellular matrix assembly. J. Cell Commun. Signal. 3, 239–246 (2009).
Weaver, M. S., Workman, G. & Sage, E. H. The copper binding domain of SPARC mediates cell survival in vitro via interaction with integrin β1 and activation of integrin-linked kinase. J. Biol. Chem. 283, 22826–22837 (2008).
Shweke, N. et al. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-β activation and cell infiltration. Am. J. Pathol. 173, 631–642 (2008).
Huang, L. et al. Transglutaminase inhibition ameliorates experimental diabetic nephropathy. Kidney Int. 76, 383–394 (2009).
He, W., Kang, Y. S., Dai, C. & Liu, Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J. Am. Soc. Nephrol. 22, 90–103 (2011).
Sharma, S., Sirin, Y. & Susztak, K. The story of Notch and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 20, 56–61 (2011).
Bielesz, B. et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J. Clin. Invest. 120, 4040–4054 (2010).
Higgins, D. F. et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J. Clin. Invest. 117, 3810–3820 (2007).
Sun, S. et al. Hypoxia-inducible factor-1α induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 75, 1278–1287 (2009).
Kume, S. et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Invest. 120, 1043–1055 (2010).
Jiang, M., Liu, K., Luo, J. & Dong, Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am. J. Pathol. 176, 1181–1192 (2010).
Koesters, R. et al. Tubular overexpression of transforming growth factor-β1 induces autophagy and fibrosis but not mesenchymal transition of renal epithelial cells. Am. J. Pathol. 177, 632–643 (2010).
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Wang, S. et al. Renal bone morphogenetic protein-7 protects against diabetic nephropathy. J. Am. Soc. Nephrol. 17, 2504–2512 (2006).
Tan, X., He, W. & Liu, Y. Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy. Kidney Int. 76, 1248–1257 (2009).
de Zeeuw, D. et al. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet 376, 1543–1551 (2010).
Mirkovic, K., van den Born, J., Navis, G. & de Borst, M. H. Vitamin D in chronic kidney disease: new potential for intervention. Curr. Drug Targets 12, 42–53 (2011).
Wang, X. et al. Mice lacking the matrix metalloproteinase-9 gene reduce renal interstitial fibrosis in obstructive nephropathy. Am. J. Physiol. Renal Physiol. 299, F973–F982 (2010).
Cheng, S., Pollock, A. S., Mahimkar, R., Olson, J. L. & Lovett, D. H. Matrix metalloproteinase 2 and basement membrane integrity: a unifying mechanism for progressive renal injury. FASEB J. 20, 1898–1900 (2006).
Zeisberg, M. et al. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 3, e100 (2006).
Li, J. et al. Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes 59, 2612–2624 (2010).
Lin, S. L. et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am. J. Pathol. 178, 911–923 (2011).
Venkatachalam, M. A. et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am. J. Physiol. Renal Physiol. 298, F1078–F1094 (2010).
Kelly, K. J., Burford, J. L. & Dominguez, J. H. Postischemic inflammatory syndrome: a critical mechanism of progression in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 297, F923–F931 (2009).
Guo, Z. J. et al. Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signaling pathway. Antioxid. Redox Signal. 10, 1699–1712 (2008).
Wilkinson, L. et al. Loss of renal microvascular integrity in postnatal Crim1 hypomorphic transgenic mice. Kidney Int. 76, 1161–1171 (2009).
Mimura, I. & Nangaku, M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat. Rev. Nephrol. 6, 667–678 (2010).
Touyz, R. M. & Briones, A. M. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens. Res. 34, 5–14 (2011).
Fine, L. G. & Norman, J. T. Chronic hypoxia as a mechanism of progression of chronic kidney diseases: from hypothesis to novel therapeutics. Kidney Int. 74, 867–872 (2008).
Gunaratnam, L. & Bonventre, J. V. HIF in kidney disease and development. J. Am. Soc. Nephrol. 20, 1877–1887 (2009).
D'Agati, V. & Schmidt, A. M. RAGE and the pathogenesis of chronic kidney disease. Nat. Rev. Nephrol. 6, 352–360 (2010).
Negre-Salvayre, A., Coatrieux, C., Ingueneau, C. & Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 153, 6–20 (2008).
Zhou, L. L. et al. Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int. 76, 1148–1160 (2009).
Shi, X. Y. et al. Advanced oxidation protein products promote inflammation in diabetic kidney through activation of renal nicotinamide adenine dinucleotide phosphate oxidase. Endocrinology 149, 1829–1839 (2008).
Daroux, M. et al. Advanced glycation end-products: implications for diabetic and non-diabetic nephropathies. Diabetes Metab. 36, 1–10 (2010).
Shanmugam, N. et al. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes 57, 879–888 (2008).
Bechtel, W. et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 16, 544–550 (2010).
Hu, K., Mars, W. M. & Liu, Y. Novel actions of tissue-type plasminogen activator in chronic kidney disease. Front. Biosci. 13, 5174–5186 (2008).
Acknowledgements
I apologize to all colleagues whose important findings could not be cited due to space limitations. Our works described in this Review were supported by the National Institutes of Health grants DK064005 and DK071040.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7, 684–696 (2011). https://doi.org/10.1038/nrneph.2011.149
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2011.149
This article is cited by
-
The pathogenic role of succinate-SUCNR1: a critical function that induces renal fibrosis via M2 macrophage
Cell Communication and Signaling (2024)
-
Fisetin ameliorates fibrotic kidney disease in mice via inhibiting ACSL4-mediated tubular ferroptosis
Acta Pharmacologica Sinica (2024)
-
The correlation of interstitial change with renal prognosis in patients with myeloperoxidase-ANCA-associated glomerulonephritis: a single-center retrospective analysis
Clinical Rheumatology (2024)
-
Analysis of the potential biological mechanisms of diosmin against renal fibrosis based on network pharmacology and molecular docking approach
BMC Complementary Medicine and Therapies (2023)
-
UCP1 alleviates renal interstitial fibrosis progression through oxidative stress pathway mediated by SIRT3 protein stability
Journal of Translational Medicine (2023)