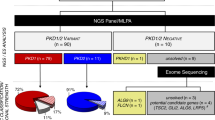
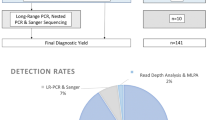
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is a common nephropathy caused by mutations in either PKD1 or PKD2. Mutations in PKD1 account for ∼85% of cases and cause more severe disease than mutations in PKD2. Diagnosis of ADPKD before the onset of symptoms is usually performed using renal imaging by either ultrasonography, CT or MRI. In general, these modalities are reliable for the diagnosis of ADPKD in older individuals. However, molecular testing can be valuable when a definite diagnosis is required in young individuals, in individuals with a negative family history of ADPKD, and to facilitate preimplantation genetic diagnosis. Although linkage-based diagnostic approaches are feasible in large families, direct mutation screening is generally more applicable. As ADPKD displays a high level of allelic heterogeneity, complete screening of both genes is required. Consequently, such screening approaches are expensive. Screening of individuals with ADPKD detects mutations in up to 91% of cases. However, only ∼65% of patients have definite mutations with ∼26% having nondefinite changes that require further evaluation. Collation of known variants in the ADPKD mutation database and systematic scoring of nondefinite variants is increasing the diagnostic value of molecular screening. Genic information can be of prognostic value and recent investigation of hypomorphic PKD1 alleles suggests that allelic information may also be valuable in some atypical cases. In the future, when effective therapies are developed for ADPKD, molecular testing may become increasingly widespread. Rapid developments in DNA sequencing may also revolutionize testing.
Key Points
-
Molecular diagnostics is available and increasingly informative in autosomal dominant polycystic kidney disease (ADPKD)
-
Determining the disease status of potential living, related donors is where molecular diagnostics is most valuable at present
-
A molecular diagnosis can clarify the disease status in patients with a negative family history and/or unusually mild or severe polycystic kidney disease
-
Determining whether a family carries mutations in PKD1 or PKD2 is of prognostic value
-
Hypomorphic PKD1 alleles can significantly modify the ADPKD phenotype and the identification of specific alleles may be of prognostic value, especially in early-onset ADPKD
-
As therapies for ADPKD are developed, molecular testing will likely become increasingly valuable
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Harris, P. C. & Torres, V. E. in GeneReviews at GeneTests: Medical Genetics Information Resource [database online]. Copyright, University of Washginton, Seattle. 1997–2008 [online], (2008).
Torres, V. E., Harris, P. C. & Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 369, 1287–1301 (2007).
Dalgaard, O. Z. Bilateral polycystic disease of the kidneys: A follow-up of two hundred and eighty-four patients and their families. Acta Med. Scand. Suppl. 328, 1–255 (1957).
Iglesias, C. G. et al. Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota. Am. J. Kidney Dis. 2, 630–639 (1983).
US Renal Data System. (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2007).
Belz, M. M. et al. Familial clustering of ruptured intracranial aneurysms in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 38, 770–776 (2001).
Rossetti, S. et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet 361, 2196–2201 (2003).
Pirson, Y., Chauveau, D. & Torres, V. Management of cerebral aneurysms in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 13, 269–276 (2002).
Grantham, J. J. et al. Volume progression in polycystic kidney disease. N. Engl. J. Med. 354, 2122–2130 (2006).
Hateboer, N. et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet 353, 103–107 (1999).
Shamshirsaz, A. et al. Autosomal-dominant polycystic kidney disease in infancy and childhood: progression and outcome. Kidney Int. 68, 2218–2224 (2005).
Zerres, K., Rudnik-Schöneborn, S., Deget, F. & German Working Group on Paediatric Nephrology. Childhood onset autosomal dominant polycystic kidney disease in sibs: clinical picture and recurrence risk. J. Med. Genet. 30, 583–588 (1993).
European Polycystic Kidney Disease Consortium. The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell 77, 881–894 (1994).
Mochizuki, T. et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 272, 1339–1342 (1996).
Rossetti, S. et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 18, 2143–2160 (2007).
Harris, P. C. et al. Cyst number but not the rate of cystic growth is associated with the mutated gene in ADPKD. J. Am. Soc. Nephrol. 17, 3013–3019 (2006).
Torra, R. et al. Increased prevalence of polycystic kidney disease type 2 among elderly polycystic patients. Am. J. Kidney Dis. 36, 728–734 (2000).
Barua, M. et al. Family history of renal disease severity predicts the mutated gene in ADPKD. J. Am. Soc. Nephrol. 20, 1833–1838 (2009).
Dicks, E. et al. Incident renal events and risk factors in autosomal dominant polycystic kidney disease: a population and family-based cohort followed for 22 years. Clin. J. Am. Soc. Nephrol. 1, 710–717 (2006).
Rossetti, S. et al. An Olmsted County population-based study indicates that PKD2 is more common than previously described [abstract]. J. Am. Soc. Nephrol. 18, 365A (2007).
Geberth, S., Ritz, E., Zeier, M. & Stier, E. Anticipation of age at renal death in autosomal dominant polycystic kidney disease (ADPKD)? Nephrol. Dial. Transplant. 10, 1603–1606 (1995).
Gabow, P. A. Autosomal dominant polycystic kidney disease - more than a renal disease. Am. J. Kidney Dis. 16, 403–413 (1990).
Reed, B. Y. et al. Variation in age at ESRD in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 51, 173–183 (2008).
Hughes, J. et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 10, 151–160 (1995).
International Polycystic Kidney Disease Consortium. Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. Cell 81, 289–298 (1995).
Loftus, B. J. et al. Genome duplications and other features in 12 Mb of DNA sequence from human chromosome 16p and 16q. Genomics 60, 295–308 (1999).
Martin, J. et al. The sequence and analysis of duplication-rich human chromosome 16. Nature 432, 988–994 (2004).
Phakdeekitcharoen, B., Watnick, T. J. & Germino, G. G. Mutation analysis of the entire replicated portion of PKD1 using genomic DNA samples. J. Am. Soc. Nephrol. 12, 955–963 (2001).
Rossetti, S. et al. A complete mutation screen of the ADPKD genes by DHPLC. Kidney Int. 61, 1588–1599 (2002).
Veldhuisen, B. et al. A spectrum of mutations in the second gene for autosomal dominant polycystic kidney disease (PKD2). Am. J. Hum. Genet. 61, 547–555 (1997).
Sandford, R. et al. Comparative analysis of the polycystic kidney disease 1 (PKD1) gene reveals an integral membrane glycoprotein with multiple evolutionary conserved domains. Hum. Mol. Genet. 6, 1483–1489 (1997).
Fliegauf, M., Benzing, T. & Omran, H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell. Biol. 8, 880–893 (2007).
Pazour, G. J., San Agustin, J. T., Follit, J. A., Rosenbaum, J. L. & Witman, G. B. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr. Biol. 12, R378–R380 (2002).
Yoder, B. K., Hou, X. & Guay-Woodford, L. M. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 13, 2508–2516 (2002).
Nauli, S. M. et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 33, 129–137 (2003).
Sharif-Naeini, R. et al. Polycystin-1 and -2 dosage regulates pressure sensing. Cell 139, 587–596 (2009).
Battini, L. et al. Loss of polycystin-1 causes centrosome amplification and genomic instability. Hum. Mol. Genet. 17, 2819–2833 (2008).
Li, X. et al. Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat. Cell Biol. 7, 1202–1212 (2005).
Happé, H. et al. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum. Mol. Genet. 18, 2532–2542 (2009).
Torres, V. E. & Harris, P. C. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 76, 149–168 (2009).
Reed, B. et al. Presence of de novo mutations in autosomal dominant polycystic kidney disease patients without family history. Am. J. Kidney Dis. 52, 1042–1050 (2008).
Rossetti, S. et al. Mutation analysis of the entire PKD1 gene: genetic and diagnostic implications. Am. J. Hum. Genet. 68, 46–63 (2001).
Pei, Y. et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J. Am. Soc. Nephrol. 20, 205–212 (2009).
Ravine, D. et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343, 824–827 (1994).
Nascimento, A. B. et al. Rapid MR imaging detection of renal cysts: age-based standards. Radiology 221, 628–632 (2001).
Carrim, Z. I. & Murchison, J. T. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin. Radiol. 58, 626–629 (2003).
Huang, E. et al. DNA testing for live kidney donors at risk for autosomal dominant polycystic kidney disease. Transplantation 87, 133–137 (2009).
Blumenfeld, J. D. Pretransplant genetic testing of live kidney donors at risk for autosomal dominant polycystic kidney disease. Transplantation 87, 6–7 (2009).
Edghill, E. L., Bingham, C., Ellard, S. & Hattersley, A. T. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J. Med. Genet. 43, 84–90 (2006).
Faguer, S. et al. Massively enlarged polycystic kidneys in monozygotic twins with TCF2/HNF-1beta (hepatocyte nuclear factor-1beta) heterozygous whole-gene deletion. Am. J. Kidney Dis. 50, 1023–1027 (2007).
Heidet, L. et al. Spectrum of HNF1B mutations in a cohort of patients harboring renal diseases [abstract]. J. Am. Soc. Nephrol. 20, 773A (2009).
Davila, S. et al. Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat. Genet. 36, 575–577 (2004).
Drenth, J. P., te Morsche, R. H., Smink, R., Bonifacino, J. S. & Jansen, J. B. Germline mutations in PRKCSH are associated with autosomal dominant polycystic liver disease. Nat. Genet. 33, 345–347 (2003).
Li, A. et al. Mutations in PRKCSH cause isolated autosomal dominant polycystic liver disease. Am. J. Hum. Genet. 72, 691–703 (2003).
Adeva, M. et al. Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD). Medicine 85, 1–21 (2006).
Peral, B. et al. Evidence of linkage disequilibrium in the Spanish polycystic kidney disease 1 population. Am. J. Hum. Genet. 54, 899–908 (1994).
Zhao, X. et al. Molecular diagnostics in autosomal dominant polycystic kidney disease: utility and limitations. Clin. J. Am. Soc. Nephrol. 8, 146–152 (2008).
De Rycke, M. et al. PGD for autosomal dominant polycystic kidney disease type 1. Mol. Hum. Reprod. 11, 65–71 (2005).
Tan, Y. C. et al. Novel method for genomic analysis of PKD1 and PKD2 mutations in autosomal dominant polycystic kidney disease. Hum. Mutat. 30, 264–273 (2009).
Garcia-Gonzalez, M. A. et al. Evaluating the clinical utility of a molecular genetic test for polycystic kidney disease. Mol. Genet. Metab. 92, 160–167 (2007).
PKD Foundation Autosomal Dominant Polycystic Kidney Disease: Mutation Database [online], (2010).
Magistroni, R. et al. Genotype-renal function correlation in type 2 autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 14, 1164–1174 (2003).
Rossetti, S. et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 75, 848–855 (2009).
Consugar, M. B. et al. Characterization of large rearrangements in autosomal dominant polycystic kidney disease and the PKD1/TSC2 contiguous gene syndrome. Kidney Int. 74, 1468–1479 (2008).
Brook-Carter, P. T. et al. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease—a contiguous gene syndrome. Nat. Genet. 8, 328–332 (1994).
Sampson, J. R. et al. Renal cystic disease in tuberous sclerosis: role of the polycystic kidney disease 1 gene. Am. J. Hum. Genet. 61, 843–851 (1997).
Koulen, P. et al. Polycystin-2 is an intracellular calcium release channel. Nat. Cell Biol. 4, 191–197 (2002).
Qian, F. et al. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc. Natl Acad. Sci. USA 99, 16981–16986 (2002).
Forman, J. R., Qamar, S., Paci, E., Sandford, R. N. & Clarke, J. The remarkable mechanical strength of polycystin-1 supports a direct role in mechanotransduction. J. Mol. Biol. 349, 861–871 (2005).
Ma, L., Xu, M., Forman, J. R., Clarke, J. & Oberhauser, A. F. Naturally occurring mutations alter the stability of polycystin-1 PKD domains. J. Biol. Chem. 284, 32942–32949 (2009).
Qian, F., Wei, W., Germino, G. & Oberhauser, A. The nanomechanics of polycystin-1 extracellular region. J. Biol. Chem. 280, 40723–40730 (2005).
Consugar, M. B. et al. Characteristics of CRISP ADPKD patients with no detected base-pair mutations [abstract]. J. Am. Soc. Nephrol. 19, 125A (2008).
King, K., Flinter, F. A., Nihalani, V. & Green, P. M. Unusual deep intronic mutations in the COL4A5 gene cause X linked Alport syndrome. Hum. Genet. 111, 548–554 (2002).
Gorlov, I. P., Gorlova, O. Y., Frazier, M. L. & Amos, C. I. Missense mutations in hMLH1 and hMSH2 are associated with exonic splicing enhancers. Am. J. Hum. Genet. 73, 1157–1161 (2003).
Daoust, M. C., Reynolds, D. M., Bichet, D. G. & Somlo, S. Evidence for a third genetic locus for autosomal dominant polycystic kidney disease. Genomics 25, 733–736 (1995).
de Almeida, S. et al. Autosomal dominant polycystic kidney disease: evidence for the existence of a third locus in a Portuguese family. Hum. Genet. 96, 83–88 (1995).
Consugar, M. et al. PKD3 revisited with improved PKD1 and PKD2 haplotyping and mutation screening [abstract]. J. Am. Soc. Nephrol. 16, 358A (2005).
Connor, A. et al. Mosaicism in autosomal dominant polycystic kidney disease revealed by genetic testing to enable living related renal transplantation. Am. J. Transplant. 8, 232–237 (2008).
Rossetti, S. et al. The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J. Am. Soc. Nephrol. 13, 1230–1237 (2002).
Bergmann, C. et al. Spectrum of mutations in the gene for autosomal recessive polycystic kidney disease (ARPKD/PKHD1). J. Am. Soc. Nephrol. 14, 76–89 (2003).
Jiang, S. T. et al. Defining a link with autosomal-dominant polycystic kidney disease in mice with congenitally low expression of Pkd1. Am. J. Pathol. 168, 205–220 (2006).
Kim, I. et al. Polycystin-2 expression is regulated by a PC2-binding domain in the intracellular portion of fibrocystin. J. Biol. Chem. 283, 31559–31566 (2008).
Lantinga-van Leeuwen, I. S. et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum. Mol. Genet. 13, 3069–3077 (2004).
Sandford, R. N. The diversity of PKD1 alleles: implications for disease pathogenesis and genetic counseling. Kidney Int. 75, 765–767 (2009).
Fain, P. R. et al. Modifier genes play a significant role in the phenotypic expression of PKD1. Kidney Int. 67, 1256–1267 (2005).
Paterson, A. D. et al. Progressive loss of renal function is an age-dependent heritable trait in type 1 autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 16, 755–762 (2005).
Tucker, T., Marra, M. & Friedman, J. M. Massively parallel sequencing: the next big thing in genetic medicine. Am. J. Hum. Genet. 85, 142–154 (2009).
Mardis, E. R. The impact of next-generation sequencing technology on genetics. Trends Genet. 24, 133–141 (2008).
Voelkerding, K. V., Dames, S. A. & Durtschi, J. D. Next-generation sequencing: from basic research to diagnostics. Clin. Chem. 55, 641–658 (2009).
Mardis, E. R. Next-generation DNA sequencing methods. Annu. Rev. Genomics Hum. Genet. 9, 387–402 (2008).
Eid, J. et al. Real-time DNA sequencing from single polymerase molecules. Science 323, 133–138 (2009).
Shendure, J. & Ji, H. Next-generation DNA sequencing. Nat. Biotechnol. 26, 1135–1145 (2008).
Branton, D. et al. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 26, 1146–1153 (2008).
Ravine, D., Gibson, R. N., Donlan, J. & Sheffield, L. J. An ultrasound renal cyst prevalence survey: specificity data for inherited renal cystic diseases. Am. J. Kidney Dis. 22, 803–807 (1993).
Grantham, R. Amino acid difference formula to help explain protein evolution. Science 185, 862–864 (1974).
Bork Group and Sunyaev Lab. PolyPhen: prediction of functional effect on human nsSNPs [online], (2009).
J. Craig Venter Institute. SIFT [online], (2009).
International Agency for Research on Cancer. Align GVGD [online], (2009).
Desmet, F. O. et al. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acid Research [online], (2009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Harris, P., Rossetti, S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6, 197–206 (2010). https://doi.org/10.1038/nrneph.2010.18
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.18
This article is cited by
-
Exploring the clinical and genetical spectrum of ADPKD in Chile to assess ProPKD score as a risk prediction tool
Translational Medicine Communications (2023)
-
Generation of heterozygous PKD1 mutant pigs exhibiting early-onset renal cyst formation
Laboratory Investigation (2022)
-
Monkeys mutant for PKD1 recapitulate human autosomal dominant polycystic kidney disease
Nature Communications (2019)
-
PKD1 Duplicated regions limit clinical Utility of Whole Exome Sequencing for Genetic Diagnosis of Autosomal Dominant Polycystic Kidney Disease
Scientific Reports (2019)
-
A novel splicing mutation in the PKD1 gene causes autosomal dominant polycystic kidney disease in a Chinese family: a case report
BMC Medical Genetics (2018)