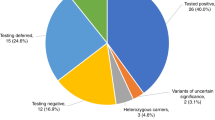
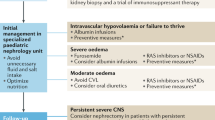
Abstract
Patients with some hereditary nephropathies—including autosomal dominant polycystic kidney disease (ADPKD), Fabry disease and Alport syndrome—can progress to end-stage renal disease (ESRD) and are candidates for kidney transplantation. When considering whether a potential living donor is appropriate for a particular patient, clinicians should be aware of the increased risk of adverse outcomes for the donor and the recipient. Renal transplantation from a living related donor is not contraindicated in most nephropathies that have an autosomal recessive mode of inheritance (for example, autosomal recessive polycystic kidney disease and cystinosis). Renal transplant recipients with ADPKD, however, should only receive a kidney from a related donor if the disease has been excluded in the donor by imaging and/or genetic testing. Potential living related donors for patients with Alport syndrome should be evaluated carefully for the presence of microhematuria and microalbuminuria before a decision is made to perform transplantation, and mothers or heterozygous sisters of affected male recipients with X-linked Alport syndrome should be informed about the possible long-term increased risk of renal dysfunction associated with donation. Most patients with atypical hemolytic uremic syndrome should not receive a kidney transplant from a living donor because there is a high risk of disease recurrence and graft loss.
Key Points
-
Living donor kidney transplantation is often presented as the best option for patients awaiting renal transplantation, but patients whose renal failure is the result of an inherited disease might not be suitable candidates for living related transplantation
-
The possible occurrence of the same disease in the related living donor should be excluded before living donor transplantation is performed in a patient with a hereditary nephropathy
-
For living donor transplantation to be suitable for a particular patient, the risk of graft loss should not be higher than it would be if the patient received a graft from a deceased donor
-
Individuals with autosomal dominant polycystic kidney disease requiring renal transplantation should only receive a kidney from a relative if the disease has been excluded in that relative; if imaging data is equivocal, the potential living related donor should undergo molecular testing
-
Female heterozygotes for X-linked Alport syndrome who are considering donating a kidney should be informed about the long-term increased risk of renal dysfunction associated with kidney donation
-
Living donor kidney transplantation is contraindicated in patients with atypical hemolytic uremic syndrome because of the high risk of recurrence and the associated high risk of graft loss
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Davis, C. L. & Delmonico, F. L. Living-donor kidney transplantation: a review of the current practices for the live donor. J. Am. Soc. Nephrol. 16, 2098–2110 (2005).
Mange, K. C., Joffe, M. M. & Feldman, H. I. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N. Engl. J. Med. 344, 726–731 (2001).
Kasiske, B. L. et al. The evaluation of living renal transplant donors: clinical practice guidelines. Ad Hoc Clinical Practice Guidelines Subcommittee of the Patient Care and Education Committee of the American Society of Transplant Physicians. J. Am. Soc. Nephrol. 7, 2288–2313 (1996).
Donne, R. L. et al. Recurrence of hemolytic uremic syndrome after live related renal transplantation associated with subsequent de novo disease in the donor. Am. J. Kidney Dis. 40, E22 (2002).
Grantham, J. J. Clinical practice. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 359, 1477–1485 (2008).
Torres, V. E., Harris, P. C. & Pirson, Y. Autosomal dominant polycystic kidney disease. Lancet 369, 1287–1301 (2007).
Gallagher, A. R., Germino, G. G. & Somlo, S. Molecular advances in autosomal dominant polycystic kidney disease. Adv. Chronic Kidney Dis. 17, 118–130 (2010).
Hateboer, N. et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353, 103–107 (1999).
Bear, J. C. et al. Age at clinical onset and at ultrasonographic detection of adult polycystic kidney disease: data for genetic counselling. Am. J. Med. Genet. 18, 45–53 (1984).
Nicolau, C. et al. Autosomal dominant polycystic kidney disease types 1 and 2: assessment of US sensitivity for diagnosis. Radiology 213, 273–276 (1999).
Ravine, D. et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343, 824–827 (1994).
Pei, Y. et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J. Am. Soc. Nephrol. 20, 205–212 (2009).
Zand, M. S. et al. Screening a living kidney donor for polycystic kidney disease using heavily T2-weighted MRI. Am. J. Kidney Dis. 37, 612–619 (2001).
Blumenfeld, J. D. Pretransplant genetic testing of live kidney donors at risk for autosomal dominant polycystic kidney disease. Transplantation 87, 6–7 (2009).
Huang, E. et al. DNA testing for live kidney donors at risk for autosomal dominant polycystic kidney disease. Transplantation 87, 133–137 (2009).
Rossetti, S. et al. A complete mutation screen of the ADPKD genes by DHPLC. Kidney Int. 61, 1588–1599 (2002).
Veldhuisen, B. et al. A spectrum of mutations in the second gene for autosomal dominant polycystic kidney disease (PKD2). Am. J. Hum. Genet. 61, 547–555 (1997).
Hannig, V. L., Erickson, S. M. & Phillips, J. A. 3rd. Utilization and evaluation of living-related donors for patients with adult polycystic kidney disease. Am. J. Med. Genet. 44, 409–412 (1992).
Harris, P. C. & Rossetti, S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 6, 197–206 (2010).
Heidet, L. & Gubler, M. C. The renal lesions of Alport syndrome. J. Am. Soc. Nephrol. 20, 1210–1215 (2009).
Kashtan, C. E. Alport syndrome and thin glomerular basement membrane disease. J. Am. Soc. Nephrol. 9, 1736–1750 (1998).
Tryggvason, K., Zhou, J., Hostikka, S. L. & Shows, T. B. Molecular genetics of Alport syndrome. Kidney Int. 43, 38–44 (1993).
Barker, D. F. et al. Identification of mutations in the COL4A5 collagen gene in Alport syndrome. Science 248, 1224–1227 (1990).
Jais, J. P. et al. X-linked Alport syndrome: natural history and genotype-phenotype correlations in girls and women belonging to 195 families: a “European Community Alport Syndrome Concerted Action” study. J. Am. Soc. Nephrol. 14, 2603–2610 (2003).
Martin, P. et al. High mutation detection rate in the COL4A5 collagen gene in suspected Alport syndrome using PCR and direct DNA sequencing. J. Am. Soc. Nephrol. 9, 2291–2301 (1998).
Heiskari, N. et al. Identification of 17 mutations in ten exons in the COL4A5 collagen gene, but no mutations found in four exons in COL4A6: a study of 250 patients with hematuria and suspected of having Alport syndrome. J. Am. Soc. Nephrol. 7, 702–709 (1996).
Feingold, J. et al. Genetic heterogeneity of Alport syndrome. Kidney Int. 27, 672–677 (1985).
Heidet, L. et al. Structure of the human type IV collagen gene COL4A3 and mutations in autosomal Alport syndrome. J. Am. Soc. Nephrol. 12, 97–106 (2001).
Mochizuki, T. et al. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat. Genet. 8, 77–81 (1994).
Torra, R., Tazon-Vega, B., Ars, E. & Ballarin, J. Collagen type IV (α3- α4) nephropathy: from isolated haematuria to renal failure. Nephrol. Dial. Transplant. 19, 2429–2432 (2004).
Pescucci, C. et al. Autosomal-dominant Alport syndrome: natural history of a disease due to COL4A3 or COL4A4 gene. Kidney Int. 65, 1598–1603 (2004).
van der Loop, F. T. et al. Autosomal dominant Alport syndrome caused by a COL4A3 splice site mutation. Kidney Int. 58, 1870–1875 (2000).
Jais, J. P. et al. X-linked Alport syndrome: natural history in 195 families and genotype-phenotype correlations in males. J. Am. Soc. Nephrol. 11, 649–657 (2000).
Flinter, F. Alport's syndrome. J. Med. Genet. 34, 326–330 (1997).
Colville, D. et al. Ocular manifestations of autosomal recessive Alport syndrome. Ophthalmic Genet. 18, 119–128 (1997).
Kashtan, C. E. Women with Alport syndrome: risks and rewards of kidney donation. Nephrol. Dial. Transplant. 24, 1369–1370 (2009).
Gross, O., Weber, M., Fries, J. W. & Muller, G. A. Living donor kidney transplantation from relatives with mild urinary abnormalities in Alport syndrome: long-term risk, benefit and outcome. Nephrol. Dial. Transplant. 24, 1626–1630 (2009).
Badenas, C. et al. Mutations in the COL4A4 and COL4A3 genes cause familial benign hematuria. J. Am. Soc. Nephrol. 13, 1248–1254 (2002).
Buzza, M., Wilson, D. & Savige, J. Segregation of hematuria in thin basement membrane disease with haplotypes at the loci for Alport syndrome. Kidney Int. 59, 1670–1676 (2001).
Voskarides, K. et al. COL4A3/COL4A4 mutations producing focal segmental glomerulosclerosis and renal failure in thin basement membrane nephropathy. J. Am. Soc. Nephrol. 18, 3004–3016 (2007).
Kashtan, C. E. Alport syndrome. An inherited disorder of renal, ocular, and cochlear basement membranes. Medicine (Baltimore) 78, 338–360 (1999).
Kashtan, C. E. Renal transplantation in patients with Alport syndrome. Pediatr. Transplant. 10, 651–657 (2006).
Ding, J., Zhou, J., Tryggvason, K. & Kashtan, C. E. COL4A5 deletions in three patients with Alport syndrome and posttransplant antiglomerular basement membrane nephritis. J. Am. Soc. Nephrol. 5, 161–168 (1994).
Kashtan, C. E., Butkowski, R. J., Kleppel, M. M., First, M. R. & Michael, A. F. Posttransplant anti-glomerular basement membrane nephritis in related males with Alport syndrome. J. Lab. Clin. Med. 116, 508–515 (1990).
Branton, M. H. et al. Natural history of Fabry renal disease: influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 81, 122–138 (2002).
MacDermot, K. D., Holmes, A. & Miners, A. H. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J. Med. Genet. 38, 769–775 (2001).
Galanos, J. et al. Clinical features of Fabry's disease in Australian patients. Intern. Med. J. 32, 575–584 (2002).
Nakao, S. et al. Fabry disease: detection of undiagnosed hemodialysis patients and identification of a “renal variant” phenotype. Kidney Int. 64, 801–807 (2003).
Popli, S. et al. Involvement of renal allograft by Fabry's disease. Am. J. Nephrol. 7, 316–318 (1987).
Loirat, C. & Niaudet, P. The risk of recurrence of hemolytic uremic syndrome after renal transplantation in children. Pediatr. Nephrol. 18, 1095–1101 (2003).
Bresin, E. et al. Outcome of renal transplantation in patients with non-Shiga toxin-associated hemolytic uremic syndrome: prognostic significance of genetic background. Clin. J. Am. Soc. Nephrol. 1, 88–99 (2006).
Noris, M. & Remuzzi, G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 361, 1676–1687 (2009).
Caprioli, J. et al. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood 108, 1267–1279 (2006).
Neumann, H. P. et al. Haemolytic uraemic syndrome and mutations of the factor H gene: a registry-based study of German speaking countries. J. Med. Genet. 40, 676–681 (2003).
Sellier-Leclerc, A. L. et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 18, 2392–2400 (2007).
Loirat, C. & Fremeaux-Bacchi, V. Hemolytic uremic syndrome recurrence after renal transplantation. Pediatr. Transplant. 12, 619–629 (2008).
Le Quintrec, M. et al. Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am. J. Transplant. 8, 1694–1701 (2008).
Kavanagh, D. et al. Mutations in complement factor I predispose to development of atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 16, 2150–2155 (2005).
Saland, J. M., Ruggenenti, P. & Remuzzi, G. Liver-kidney transplantation to cure atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 20, 940–949 (2009).
Fremeaux-Bacchi, V. et al. Genetic and functional analyses of membrane cofactor protein (CD46) mutations in atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 17, 2017–2025 (2006).
Richards, A. et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc. Natl Acad. Sci. USA 100, 12966–12971 (2003).
Chan, M. R. et al. Recurrent atypical hemolytic uremic syndrome associated with factor I mutation in a living related renal transplant recipient. Am. J. Kidney Dis. 53, 321–326 (2009).
Levy, M. & Feingold, J. Estimating prevalence in single-gene kidney diseases progressing to renal failure. Kidney Int. 58, 925–943 (2000).
Latta, K. & Brodehl, J. Primary hyperoxaluria type I. Eur. J. Pediatr. 149, 518–522 (1990).
Amoroso, A. et al. AGXT gene mutations and their influence on clinical heterogeneity of type 1 primary hyperoxaluria. J. Am. Soc. Nephrol. 12, 2072–2079 (2001).
Watts, R. W. Primary hyperoxaluria type I. QJM 87, 593–600 (1994).
Brinkert, F. et al. Transplantation procedures in children with primary hyperoxaluria type 1: outcome and longitudinal growth. Transplantation 87, 1415–1421 (2009).
Millan, M. T. et al. One hundred percent patient and kidney allograft survival with simultaneous liver and kidney transplantation in infants with primary hyperoxaluria: a single-center experience. Transplantation 76, 1458–1463 (2003).
Saborio, P. & Scheinman, J. I. Transplantation for primary hyperoxaluria in the United States. Kidney Int. 56, 1094–1100 (1999).
Cochat, P. & Scharer, K. Should liver transplantation be performed before advanced renal insufficiency in primary hyperoxaluria type 1? Pediatr. Nephrol. 7, 212–218 (1993).
Gruessner, R. W. Preemptive liver transplantation from a living related donor for primary hyperoxaluria type I. N. Engl. J. Med. 338, 1924 (1998).
Monico, C. G., Rossetti, S., Olson, J. B. & Milliner, D. S. Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int. 67, 1704–1709 (2005).
van Woerden, C. S. et al. Clinical implications of mutation analysis in primary hyperoxaluria type 1. Kidney Int. 66, 746–752 (2004).
Scheinman, J. I., Najarian, J. S. & Mauer, S. M. Successful strategies for renal transplantation in primary oxalosis. Kidney Int. 25, 804–811 (1984).
Astarcioglu, I. et al. Primary hyperoxaluria: simultaneous combined liver and kidney transplantation from a living related donor. Liver Transpl. 9, 433–436 (2003).
Rosenblatt, G. S., Jenkins, R. D. & Barry, J. M. Treatment of primary hyperoxaluria type 1 with sequential liver and kidney transplants from the same living donor. Urology 68, 427.e7–427.e8 (2006).
Jungraithmayr, T. C. et al. Primary focal segmental glomerulosclerosis—long-term outcome after pediatric renal transplantation. Pediatr. Transplant. 9, 226–231 (2005).
Cameron, J. S. Recurrent primary disease and de novo nephritis following renal transplantation. Pediatr. Nephrol. 5, 412–421 (1991).
Habib, R., Hebert, D., Gagnadoux, M. F. & Broyer, M. Transplantation in idiopathic nephrosis. Transplant. Proc. 14, 489–495 (1982).
Ramos, E. L. & Tisher, C. C. Recurrent diseases in the kidney transplant. Am. J. Kidney Dis. 24, 142–154 (1994).
Striegel, J. E., Sibley, R. K., Fryd, D. S. & Mauer, S. M. Recurrence of focal segmental sclerosis in children following renal transplantation. Kidney Int. Suppl. 19, S44–S50 (1986).
Niaudet, P. Genetic forms of nephrotic syndrome. Pediatr. Nephrol. 19, 1313–1318 (2004).
Weber, S. et al. NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int. 66, 571–579 (2004).
Boute, N. et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat. Genet. 24, 349–354 (2000).
Caridi, G. et al. Prevalence, genetics, and clinical features of patients carrying podocin mutations in steroid-resistant nonfamilial focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 12, 2742–2746 (2001).
Becker-Cohen, R. et al. Recurrent nephrotic syndrome in homozygous truncating NPHS2 mutation is not due to anti-podocin antibodies. Am. J. Transplant. 7, 256–260 (2007).
Bertelli, R. et al. Recurrence of focal segmental glomerulosclerosis after renal transplantation in patients with mutations of podocin. Am. J. Kidney Dis. 41, 1314–1321 (2003).
Billing, H. et al. NPHS2 mutation associated with recurrence of proteinuria after transplantation. Pediatr. Nephrol. 19, 561–564 (2004).
Höcker, B. et al. Recurrence of proteinuria 10 years post-transplant in NPHS2-associated focal segmental glomerulosclerosis after conversion from cyclosporin A to sirolimus. Pediatr. Nephrol. 21, 1476–1479 (2006).
Ruf, R. G. et al. Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J. Am. Soc. Nephrol. 15, 722–732 (2004).
Machuca, E. et al. Clinical and epidemiological assessment of steroid-resistant nephrotic syndrome associated with the NPHS2 R229Q variant. Kidney Int. 75, 727–735 (2009).
Tsukaguchi, H. et al. NPHS2 mutations in late-onset focal segmental glomerulosclerosis: R229Q is a common disease-associated allele. J. Clin. Invest. 110, 1659–1666 (2002).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Niaudet, P. Living donor kidney transplantation in patients with hereditary nephropathies. Nat Rev Nephrol 6, 736–743 (2010). https://doi.org/10.1038/nrneph.2010.122
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.122
This article is cited by
-
Retrospective genetic analysis illustrates the spectrum of autosomal Alport syndrome in a case of living-related donor kidney transplantation
BMC Nephrology (2019)
-
Genes in FSGS: Diagnostic and Management Strategies in Children
Current Pediatrics Reports (2015)
-
Congenital nephrotic syndrome and recurrence of proteinuria after renal transplantation
Pediatric Nephrology (2014)