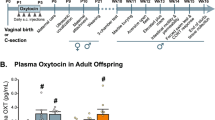
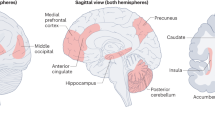
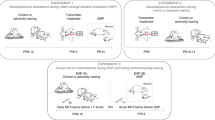
Abstract
Birth is associated with a neuroprotective, oxytocin-mediated abrupt excitatory-to-inhibitory GABA shift that is abolished in autism, and its restoration attenuates the disorder in offspring. In this Opinion article, I discuss the links between birth-related stressful mechanisms, persistent excitatory GABA actions, perturbed network oscillations and autism. I propose that birth (parturition) is a critical period that confirms, attenuates or aggravates the deleterious effects of intrauterine genetic or environmental insults.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rodríguez, J. M. et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26, 26050 (2015).
Glanowska, K. M., Burger, L. L. & Moenter, S. M. Development of gonadotropin-releasing hormone secretion and pituitary response. J. Neurosci. 34, 15060–15069 (2014).
Romero, R. & Korzeniewski, S. J. Are infants born by elective cesarean delivery without labor at risk for developing immune disorders later in life? Am. J. Obstet. Gynecol. 208, 243–246 (2013).
Cryan, J. F. & Dinan, T. G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712 (2012).
Loria, A. S., D'Angelo, G., Pollock, D. M. & Pollock, J. S. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R185–R191 (2010).
Kros, C. J., Ruppersberg, J. P. & Rüsch, A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature 394, 281–284 (1998).
Nakamura, Y. & Takahashi, T. Developmental changes in potassium currents at the rat calyx of Held presynaptic terminal. J. Physiol. 581, 1101–1112 (2007).
Kuhar, S. G. et al. Changing patterns of gene expression define four stages of cerebellar granule neuron differentiation. Development 117, 97–104 (1993).
Ratto, G. M., Bisti, S. & Chalupa, L. M. Functional development of intrinsic properties in ganglion cells of the mammalian retina. J. Neurophysiol. 78, 2895–2903 (1997).
Skaliora, I., Scobey, R. P. & Chalupa, L. M. Prenatal development of excitability in cat retinal ganglion cells: action potentials and sodium currents. J. Neurosci. 13, 313–323 (1993).
Spitzer, N. C. & Ribera, A. B. Development of electrical excitability in embryonic neurons: mechanisms and roles. J. Neurobiol. 37, 190–197 (1998).
Monyer, H., Burnashev, N., Laurie, D. J., Sakmann, B. & Seeburg, P. H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540 (1994).
Hartveit, E. et al. Localization and developmental expression of the NMDA receptor subunit NR2A in the mammalian retina. J. Comp. Neurol. 348, 570–582 (1994).
Laurie, D. J. & Seeburg, P. H. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J. Neurosci. 14, 3180–3194 (1994).
Yamada, J. et al. Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J. Physiol. 557, 829–841 (2004).
Ben-Ari, Y., Cherubini, E., Corradetti, R. & Gaiarsa, J. L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 416, 303–325 (1989).
Ben-Ari, Y., Gaiarsa, J.-L., Tyzio, R. & Khazipov, R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol. Rev. 87, 1215–1284 (2007).
Ben-Ari, Y. The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience 279, 187–219 (2014).
Crépel, V. et al. A parturition-associated nonsynaptic coherent activity pattern in the developing hippocampus. Neuron 54, 105–120 (2007).
Bonifazi, P. et al. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326, 1419–1424 (2009).
Colonnese, M. T. et al. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron 67, 480–498 (2010).
Hanganu, I. L., Ben-Ari, Y. & Khazipov, R. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J. Neurosci. 26, 6728–6736 (2006).
Torborg, C. L. & Feller, M. B. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog. Neurobiol. 76, 213–235 (2005).
Xu, H.-P. et al. An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron 70, 1115–1127 (2011).
Mooney, R., Penn, A. A., Gallego, R. & Shatz, C. J. Thalamic relay of spontaneous retinal activity prior to vision. Neuron 17, 863–874 (1996).
Ford, K. J., Félix, A. L. & Feller, M. B. Cellular mechanisms underlying spatiotemporal features of cholinergic retinal waves. J. Neurosci. 32, 850–863 (2012).
Maffei, L. & Galli-Resta, L. Correlation in the discharges of neighboring rat retinal ganglion cells during prenatal life. Proc. Natl Acad. Sci. USA 87, 2861–2864 (1990).
Roerig, B. & Feller, M. B. Neurotransmitters and gap junctions in developing neural circuits. Brain Res. Brain Res. Rev. 32, 86–114 (2000).
Rochefort, N. L. et al. Sparsification of neuronal activity in the visual cortex at eye-opening. Proc. Natl Acad. Sci. USA 106, 15049–15054 (2009).
Wong, A. B. et al. Concurrent maturation of inner hair cell synaptic Ca2+ influx and auditory nerve spontaneous activity around hearing onset in mice. J. Neurosci. 33, 10661–10666 (2013).
Barth, A. L. & Poulet, J. F. Experimental evidence for sparse firing in the neocortex. Trends Neurosci. 35, 345–355 (2012).
Wolfe, J., Houweling, A. R. & Brecht, M. Sparse and powerful cortical spikes. Curr. Opin. Neurobiol. 20, 306–312 (2010).
Dehorter, N., Vinay, L., Hammond, C. & Ben-Ari, Y. Timing of developmental sequences in different brain structures: physiological and pathological implications. Eur. J. Neurosci. 35, 1846–1856 (2012).
Hammond, C., Bergman, H. & Brown, P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 30, 357–364 (2007).
Brown, P. in Parkinson's Disease and Related Disorders (eds Riederer, P., Reichmann, H., Youdim, M. B. H. & Gerlach, M.) 27–30 (Springer, 2006).
Elsabbagh, M. et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 5, 160–179 (2012).
Khan, N. Z. et al. Autism and the grand challenges in global mental health. Autism Res. 5, 156–159 (2012).
Courchesne, E. et al. Neuron number and size in prefrontal cortex of children with autism. JAMA 306, 2001–2010 (2011).
Stoner, R. et al. Patches of disorganization in the neocortex of children with autism. N. Engl. J. Med. 370, 1209–1219 (2014).
Johnson, S. et al. Autism spectrum disorders in extremely preterm children. J. Pediatr. 156, 525–531.e2 (2010).
Shelton, J. F. et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ. Health Perspect. 122, 1103–1109 (2014).
Toro, R. et al. Key role for gene dosage and synaptic homeostasis in autism spectrum disorders. Trends Genet. 26, 363–372 (2010).
Schmeisser, M. J. et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature 486, 256–260 (2012).
Bourgeron, T. A synaptic trek to autism. Curr. Opin. Neurobiol. 19, 231–234 (2009).
Autism Genome Project Consortium et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genet. 39, 319–328 (2007).
Williams, E. L. & Casanova, M. F. Above genetics: lessons from cerebral development in autism. Transl Neurosci. 2, 106–120 (2011).
Sandin, S. et al. The familial risk of autism. JAMA 311, 1770–1777 (2014).
Ben-Ari, Y. & Spitzer, N. C. Phenotypic checkpoints regulate neuronal development. Trends Neurosci. 33, 485–492 (2010).
Spitzer, N. C. & Borodinsky, L. N. Implications of activity-dependent neurotransmitter-receptor matching. Phil. Trans. R. Soc. B 363, 1393–1399 (2008).
Komuro, H. & Rakic, P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron 17, 275–285 (1996).
Manent, J. B. et al. A noncanonical release of GABA and glutamate modulates neuronal migration. J. Neurosci. 25, 4755–4765 (2005).
Manent, J.-B., Jorquera, I., Ben-Ari, Y., Aniksztejn, L. & Represa, A. Glutamate acting on AMPA but not NMDA receptors modulates the migration of hippocampal interneurons. J. Neurosci. 26, 5901–5909 (2006).
Borodinsky, L. N. et al. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 429, 523–530 (2004).
Verhage, M. et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 287, 864–869 (2000).
Demarque, M. et al. Glutamate transporters prevent the generation of seizures in the developing rat neocortex. J. Neurosci. 24, 3289–3294 (2004).
Wang, G. X. & Poo, M.-M. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature 434, 898–904 (2005).
Ben-Ari, Y. Neuro-archaeology: pre-symptomatic architecture and signature of neurological disorders. Trends Neurosci. 31, 626–636 (2008).
Ackman, J. B. et al. Abnormal network activity in a targeted genetic model of human double cortex. J. Neurosci. 29, 313–327 (2009).
Salmi, M. et al. Tubacin prevents neuronal migration defects and epileptic activity caused by rat Srpx2 silencing in utero. Brain 136, 2457–2473 (2013).
Falace, A. et al. TBC1D24 regulates neuronal migration and maturation through modulation of the ARF6-dependent pathway. Proc. Natl Acad. Sci. USA 111, 2337–2342 (2014).
Chen, L., Bao, S., Qiao, X. & Thompson, R. F. Impaired cerebellar synapse maturation in waggler, a mutant mouse with a disrupted neuronal calcium channel γ subunit. Proc. Natl Acad. Sci. USA 96, 12132–12137 (1999).
Manent, J.-B., Wang, Y., Chang, Y., Paramasivam, M. & LoTurco, J. J. Dcx reexpression reduces subcortical band heterotopia and seizure threshold in an animal model of neuronal migration disorder. Nat. Med. 15, 84–90 (2009).
Tyzio, R. et al. Membrane potential of CA3 hippocampal pyramidal cells during postnatal development. J. Neurophysiol. 90, 2964–2972 (2003).
Rivera, C. et al. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255 (1999).
Lu, J., Karadsheh, M. & Delpire, E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J. Neurobiol. 39, 558–568 (1999).
Chabrol, F. P., Eglen, S. J. & Sernagor, E. GABAergic control of retinal ganglion cell dendritic development. Neuroscience 227, 30–43 (2012).
Leinekugel, X., Medina, I., Khalilov, I., Ben-Ari, Y. & Khazipov, R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABAA and NMDA receptors in the neonatal hippocampus. Neuron 18, 243–255 (1997).
Tyzio, R. et al. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 314, 1788–1792 (2006).
Bergqvist, L. L., Katz-Salamon, M., Hertegård, S., Anand, K. J. & Lagercrantz, H. Mode of delivery modulates physiological and behavioral responses to neonatal pain. J. Perinatol. 29, 44–50 (2009).
Mazzuca, M. et al. Newborn analgesia mediated by oxytocin during delivery. Front. Cell. Neurosci. 5, 3 (2011).
Balena, T. & Woodin, M. A. Coincident pre- and postsynaptic activity downregulates NKCC1 to hyperpolarize ECl during development. Eur. J. Neurosci. 27, 2402–2412 (2008).
Blaesse, P., Airaksinen, M. S., Rivera, C. & Kaila, K. Cation-chloride cotransporters and neuronal function. Neuron 61, 820–838 (2009).
Khalilov, I., Le Van, Q. M., Gozlan, H. & Ben-Ari, Y. Epileptogenic actions of GABA and fast oscillations in the developing hippocampus. Neuron 48, 787–796 (2005).
Huberfeld, G. et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J. Neurosci. 27, 9866–9873 (2007).
Khalilov, I., Holmes, G. L. & Ben-Ari, Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat. Neurosci. 6, 1079–1085 (2003).
Barmashenko, G., Hefft, S., Aertsen, A., Kirschstein, T. & Köhling, R. Positive shifts of the GABAA receptor reversal potential due to altered chloride homeostasis is widespread after status epilepticus. Epilepsia 52, 1570–1578 (2011).
Dzhala, V. I. et al. NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 11, 1205–1213 (2005).
Wake, H. et al. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J. Neurosci. 27, 1642–1650 (2007).
Hewitt, S. A., Wamsteeker, J. I., Kurz, E. U. & Bains, J. S. Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat. Neurosci. 12, 438–443 (2009).
Kim, J. S. et al. Chronic hyperosmotic stress converts GABAergic inhibition into excitation in vasopressin and oxytocin neurons in the rat. J. Neurosci. 31, 13312–13322 (2011).
Sarkar, J., Wakefield, S., MacKenzie, G., Moss, S. J. & Maguire, J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J. Neurosci. 31, 18198–18210 (2011).
Boulenguez, P. et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 16, 302–307 (2010).
Bos, R. et al. Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proc. Natl Acad. Sci. USA 110, 348–353 (2013).
Bonislawski, D. P., Schwarzbach, E. P. & Cohen, A. S. Brain injury impairs dentate gyrus inhibitory efficacy. Neurobiol. Dis. 25, 163–169 (2007).
Nabekura, J. et al. Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J. Neurosci. 22, 4412–4417 (2002).
Jaenisch, N., Witte, O. W. & Frahm, C. Downregulation of potassium chloride cotransporter KCC2 after transient focal cerebral ischemia. 41, e151–e159 (2010).
Gagnon, M. et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat. Med. 19, 1524–1528 (2013).
Hasbargen, T. et al. Role of NKCC1 and KCC2 in the development of chronic neuropathic pain following spinal cord injury. Ann. NY Acad. Sci. 1198, 168–172 (2010).
Lee, H. H. C. et al. Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC26. J. Biol. Chem. 282, 29777–29784 (2007).
Lee, H. H., Jurd, R. & Moss, S. J. Tyrosine phosphorylation regulates the membrane trafficking of the potassium chloride co-transporter KCC2. Mol. Cell. Neurosci. 45, 173–179 (2010).
He, L.-J. et al. Conditional deletion of Mecp2 in parvalbumin-expressing GABAergic cells results in the absence of critical period plasticity. Nat. Commun. 5, 5036 (2014).
Canitano, R. Epilepsy in autism spectrum disorders. Eur. Child Adolesc. Psychiatry 16, 61–66 (2006).
Danielsson, S., Gillberg, I. C., Billstedt, E. & Gillberg, C. Epilepsy in young adults with autism: a prospective population-based follow-up study of 120 individuals diagnosed in childhood. Epilepsia 46, 918–923 (2005).
Levisohn, P. M. The autism–epilepsy connection. Epilepsia 48, 33–35 (2007).
Tuchman, R. & Rapin, I. Epilepsy in autism. Lancet Neurol. 1, 352–358 (2002).
Spence, S. J. & Schneider, M. T. The role of epilepsy and epileptiform EEGs in autism spectrum disorders. Pediatr. Res. 65, 599–606 (2009).
Chao, H. T. et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468, 263–269 (2010).
Blundell, J. et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J. Neurosci. 30, 2115–2129 (2010).
Gogolla, N. et al. Common circuit defect of excitatory–inhibitory balance in mouse models of autism. J. Neurodev. Disord. 1, 172–181 (2009).
Rubenstein, J. L. & Merzenich, M. M. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2, 255–267 (2003).
Singer, W. Synchronization of cortical activity and its putative role in information-processing and learning. Annu. Rev. Physiol. 55, 349–374 (1993).
Lisman, J. E. et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 31, 234–242 (2008).
Pizzarelli, R. & Cherubini, E. Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast. 2011, 297153 (2011).
Brown, C., Gruber, T., Boucher, J., Rippon, G. & Brock, J. Gamma abnormalities during perception of illusory figures in autism. Cortex 41, 364–376 (2005).
van Diessen, E., Senders, J., Jansen, F. E., Boersma, M. & Bruining, H. Increased power of resting-state gamma oscillations in autism spectrum disorder detected by routine electroencephalography. Eur. Arch. Psychiatry Clin. Neurosci. http://dx.doi.org/10.1007/s00406-014-0527-3 (2014).
Marrosu, F., Marrosu, G., Rachel, M. G. & Biggio, G. Paradoxical reactions elicited by diazepam in children with classic autism. Funct. Neurol. 2, 355–361 (1987).
Wing, L. & Shah, A. Catatonia in autistic spectrum disorders. Br. J. Psychiatry 176, 357–362 (2000).
Hutton, J., Goode, S., Murphy, M., Le Couteur, A. & Rutter, M. New-onset psychiatric disorders in individuals with autism. Autism 12, 373–390 (2008).
Ungvari, G. S., White, E. & Pang, A. H. Psychopathology of catatonic speech disorders and the dilemma of catatonia: a selective review. Aust. N. Z. J. Psychiatry 29, 653–660 (1995).
Zoghbi, H. Y. & Bear, M. F. Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities. Cold Spring Harb. Perspect. Biol. 4, a009886 (2012).
Bear, M. F., Huber, K. M. & Warren, S. T. The mGluR theory of fragile X mental retardation. Trends Neurosci. 27, 370–377 (2004).
Moore, S. J. et al. A clinical study of 57 children with fetal anticonvulsant syndromes. J. Med. Genet. 37, 489–497 (2000).
Rasalam, A. D. et al. Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev. Med. Child Neurol. 47, 551–555 (2005).
Christensen, J. et al. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 309, 1696–1703 (2013).
Tyzio, R. et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science 343, 675–679 (2014).
Eftekhari, S. et al. Response to comment on “Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring”. Science 346, 176 (2014).
Döring, B. et al. Ablation of connexin43 in uterine smooth muscle cells of the mouse causes delayed parturition. J. Cell. Sci. 119, 1715–1722 (2006).
Bittman, K. S., Panzer, J. A. & Balice-Gordon, R. J. Patterns of cell–cell coupling in embryonic spinal cord studied via ballistic delivery of gap-junction-permeable dyes. J. Comp. Neurol. 477, 273–285 (2004).
Sharkey, J. T., Puttaramu, R., Word, R. A. & Olcese, J. Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J. Clin. Endocrinol. Metab. 94, 421–427 (2009).
Rash, J. E. et al. Identification of connexin36 in gap junctions between neurons in rodent locus coeruleus. Neuroscience 147, 938–956 (2007).
Buzsaki, G. & Draguhn, A. Neuronal oscillations in cortical networks. Science. 304, 1926–1929 (2004).
Owen, S. F. et al. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 500, 458–462 (2014).
Lemonnier, E. et al. A randomised controlled trial of bumetanide in the treatment of autism in children. Transl Psychiatry 2, e202 (2012).
Hadjikhani, N. et al. Improving emotional face perception in autism with diuretic bumetanide: a proof-of-concept behavioral and functional brain imaging pilot study. Autism 19, 149–157 (2015).
Lagercrantz, H. & Slotkin, T. A. The “stress” of being born. Sci. Am. 254, 100–107 (1986).
Lagercrantz, H. The birth of consciousness. Early Hum. Dev. 85, S57–S58 (2009).
Lagercrantz, H. & Bistoletti, P. Catecholamine release in the newborn infant at birth. Pediatr. Res. 11, 889–893 (1977).
Faxelius, G., Hägnevik, K., Lagercrantz, H., Lundell, B. & Irestedt, L. Catecholamine surge and lung function after delivery. Arch. Dis. Child 58, 262–266 (1983).
Padbury, J. et al. Effect of fetal adrenalectomy on catecholamine release and physiologic adaptation at birth in sheep. J. Clin. Invest. 80, 1096–1103 (1987).
Newnham, J. P. et al. Fetal catecholamine release with preterm delivery. Am. J. Obstet. Gynecol. 149, 888–893 (1984).
Greenough, A., Lagercrantz, H., Pool, J. & Dahlin, I. Plasma catecholamine levels in preterm infants. Acta Paediatr. 76, 54–59 (1987).
Olver, R. & Walters, D. Why babies don't drown at birth? Acta Paediatr. 97, 1324–1326 (2008).
Aslan, E. et al. Transient tachypnea of the newborn (TTN): a role for polymorphisms in the β-adrenergic receptor (ADRB) encoding genes? Acta Paediatr. 97, 1346–1350 (2008).
Inoue, W. et al. Noradrenaline is a stress-associated metaplastic signal at GABA synapses. Nat. Neurosci. 16, 605–612 (2013).
MacKenzie, G. & Maguire, J. Neurosteroids and GABAergic signaling in health and disease. Biomol. Concepts 4, 29–42 (2013).
Inoue, W. & Bains, J. S. Beyond inhibition: GABA synapses tune the neuroendocrine stress axis. Bioessays 36, 561–569 (2014).
Krystal, A. D., Sutherland, J. & Hochman, D. W. Loop diuretics have anxiolytic effects in rat models of conditioned anxiety. PLoS ONE 7, e35417 (2012).
Bergman, H. & Deuschl, G. Pathophysiology of Parkinson's disease: from clinical neurology to basic neuroscience and back. Mov. Disord. 17, S28–S40 (2002).
Khazipov, R. et al. Early development of neuronal activity in the primate hippocampus in utero. J. Neurosci. 21, 9770–9781 (2001).
Insel, T. R. & Young, L. J. The neurobiology of attachment. Nat. Rev. Neurosci. 2, 129–136 (2001).
Modahl, C. et al. Plasma oxytocin levels in autistic children. Biol. Psychiatry 43, 270–277 (1998).
Feldman, R. et al. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol. Psychiatry 72, 175–181 (2012).
Lee, H.-J., Caldwell, H. K., Macbeth, A. H. & Young, W. S. Behavioural studies using temporal and spatial inactivation of the oxytocin receptor. Prog. Brain Res. 170, 73–77 (2008).
Sala, M. et al. Mice heterozygous for the oxytocin receptor gene (Oxtr+/−) show impaired social behaviour but not increased aggression or cognitive inflexibility: evidence of a selective haploinsufficiency gene effect. J. Neuroendocrinol. 25, 107–118 (2013).
Lee, H.-J., Pagani, J. & Young, W. S. Using transgenic mouse models to study oxytocin's role in the facilitation of species propagation. Brain Res. 1364, 216–224 (2010).
Wahl, R. U. Could oxytocin administration during labor contribute to autism and related behavioral disorders? — A look at the literature. Med. Hypotheses 63, 456–460 (2004).
Boer, G. J. & Kruisbrink, J. Effects of continuous administration of oxytocin by an accurel device on parturition in the rat and on development and diuresis in the offspring. J. Endocrinol. 101, 121–129 (1984).
Gregory, S. G., Anthopolos, R., Osgood, C. E., Grotegut, C. A. & Miranda, M. L. Association of autism with induced or augmented childbirth in North Carolina Birth Record (1990–1998) and Education Research (1997–2007) databases. JAMA Pediatr. 167, 959–966 (2013).
Henkel, L. S. et al. Common pregnancy complication ... complicated. Texas EMS Magazine [online], (2012).
Klin, A., Lin, D. J., Gorrindo, P., Ramsay, G. & Jones, W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature 459, 257–261 (2009).
Jones, E. J., Gliga, T., Bedford, R., Charman, T. & Johnson, M. H. Developmental pathways to autism: a review of prospective studies of infants at risk. Neurosci. Biobehav. Rev. 39, 1–33 (2014).
Wallace, K. S. & Rogers, S. J. Intervening in infancy: implications for autism spectrum disorders. J. Child Psychol. Psychiatry 51, 1300–1320 (2010).
Dawson, G. et al. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics 125, e17–e23 (2010).
Evans-Rogers, D. L., Sweeney, J. K., Holden-Huchton, P. & Mullens, P. A. Short-term, intensive neurodevelopmental treatment program experiences of parents and their children with disabilities. Pediatr. Phys. Ther. 27, 61–71 (2015).
Shen, M. D. et al. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain 136, 2825–2835 (2013).
Du, L. et al. A pilot study on the combination of applied behavior analysis and bumetanide treatment for children with autism. J. Child Adolesc. Psychopharmacol. (in the press).
Acknowledgements
The author is extremely grateful to his colleagues H. Lagercrantz, H. Bruining, N. Hadjikhani and D. Ferrari for their suggestions. He is also indebted to D. Ferrari for her help in preparing the figures. The author is thankful to his many colleagues who took part in the experiments summarized in this article. Financial support for the author's investigations is acknowledged from the French National Institute for Health and Medical Research (INSERM), the Institute of Neurobiology of the Mediterranean Sea (INMED), the National Agency for Research (ANR), The Bettencourt/Schuller foundation and the biotechnology company Neurochlore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author is founder and CEO of Neurochlore, a company devoted to study autism and develop a treatment based on the regulation of intracellular chloride.
Rights and permissions
About this article
Cite this article
Ben-Ari, Y. Is birth a critical period in the pathogenesis of autism spectrum disorders?. Nat Rev Neurosci 16, 498–505 (2015). https://doi.org/10.1038/nrn3956
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3956
This article is cited by
-
Abnormal Chromatin Folding in the Molecular Pathogenesis of Epilepsy and Autism Spectrum Disorder: a Meta-synthesis with Systematic Searching
Molecular Neurobiology (2023)
-
Step by step: cells with multiple functions in cortical circuit assembly
Nature Reviews Neuroscience (2022)
-
Effect of steady-state response versus excitatory/inhibitory balance on spiking synchronization in neural networks with log-normal synaptic weight distribution
Cognitive Neurodynamics (2022)
-
Early-life oxytocin attenuates the social deficits induced by caesarean-section delivery in the mouse
Neuropsychopharmacology (2021)
-
Oxytocin administration in neonates shapes hippocampal circuitry and restores social behavior in a mouse model of autism
Molecular Psychiatry (2021)