Key Points
-
Kainate receptors are a family of glutamate-gated ion channels that have diverse roles in modulating both excitatory and inhibitory neurotransmission from a variety of pre- and postsynaptic sites throughout the brain. Postsynaptic kainate receptor currents exhibit small amplitudes but very slow decay kinetics, allowing them to contribute substantially to temporal integration during high-frequency bursts of synaptic input.
-
Approaches using gene-targeting strategies and recombinant expression systems have been unable to identify the specific receptor subunit combinations responsible for the unusual kinetics of kainate receptors observed at synapses throughout the brain.
-
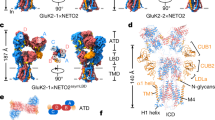
A family of CUB (complement C1R/C1s, Uegf and Bmp1) domain-containing transmembrane proteins comprising neuropilin and tolloid-like 1 (NETO1) and NETO2 were found to alter the functional properties of recombinant kainate receptors to more closely resemble those found in the CNS.
-
These newly identified kainate receptor auxiliary subunits are structurally distinct from those that modulate AMPA receptor function, including transmembrane AMPA receptor regulatory proteins, cornichons and CKAMP44 (cystine-knot AMPAR modulating protein of 44 kDa), and are more closely related to the invertebrate protein SOL-1, an auxiliary subunit of ionotropic glutamate receptors in the nematode Caenorhabditis elegans.
-
NETO1 and NETO2 co-assemble with kainate receptors to modulate a broad array of biophysical properties, including agonist affinity and efficacy, open channel probability, deactivation kinetics and rate of entry into and out of desensitization. Additionally, NETO2 promotes the forward trafficking and synaptic targeting of GluK1-containing receptors.
-
Genetic ablation of Neto1, but not Neto2, demonstrates that this auxiliary subunit co-assembles with postsynaptic kainate receptors in the hippocampus and has an essential role in mediating the slow synaptic current decay observed at this synapse without influencing measures of presynaptic activity.
Abstract
Kainate receptors are a family of ionotropic glutamate receptors whose physiological roles differ from those of other subtypes of glutamate receptors in that they predominantly serve as modulators, rather than mediators, of synaptic transmission. Neuronal kainate receptors exhibit unusually slow kinetic properties that have been difficult to reconcile with the behaviour of recombinant kainate receptors. Recently, however, the neuropilin and tolloid-like 1 (NETO1) and NETO2 proteins were identified as auxiliary kainate receptor subunits that shape both the biophysical properties and synaptic localization of these receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Contractor, A., Mulle, C. & Swanson, G. T. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 34, 154–163 (2011).
Chittajallu, R. et al. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 379, 78–81 (1996).
Castillo, P. E., Malenka, R. C. & Nicoll, R. A. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388, 182–186 (1997).
Vignes, M. & Collingridge, G. L. The synaptic activation of kainate receptors. Nature 388, 179–182 (1997). This study, with reference 3, first described postsynaptic currents mediated by kainate receptors, which were found at hippocampal mossy fibre–CA3 pyramidal cell synapses. The observed currents exhibited remarkably slow kinetics that were incongruous with the gating behaviour of recombinant kainate receptors.
Zhang, W. et al. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron 61, 385–396 (2009). This study was the first to identify the NETO proteins as potential auxiliary subunits that had a profound impact on kainate receptor functional properties.
Copits, B. A., Robbins, J. S., Frausto, S. & Swanson, G. T. Synaptic targeting and functional modulation of GluK1 kainate receptors by the auxiliary neuropilin and tolloid-like (Neto) proteins. J. Neurosci. 31, 7334–7340 (2011). This study was the first to report a role for NETO proteins in the trafficking and synaptic targeting of kainate receptors. Additionally, NETO1 and NETO2 co-assembly was found to have a bidirectional effect on GluK1 receptor function.
Straub, C. et al. Distinct functions of kainate receptors in the brain are determined by the auxiliary subunit Neto1. Nature Neurosci. 14, 866–873 (2011).
Straub, C., Zhang, W. & Howe, J. R. Neto2 modulation of kainate receptors with different subunit compositions. J. Neurosci. 31, 8078–8082 (2011).
Tang, M. et al. Neto1 Is an auxiliary subunit of native synaptic kainate receptors. J. Neurosci. 31, 10009–10018 (2011). This report and the report in reference 7 demonstrated that NETO1 confers slow gating kinetics on postsynaptic kainate receptors at the hippocampal mossy fibre synapse, thereby providing key evidence that it is an auxiliary protein for kainate receptors.
Jackson, A. C. & Nicoll, R. A. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron 70, 178–199 (2011).
Vacher, H., Mohapatra, D. P. & Trimmer, J. S. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol. Rev. 88, 1407–1447 (2008).
Straub, C. & Tomita, S. The regulation of glutamate receptor trafficking and function by TARPs and other transmembrane auxiliary subunits. Curr. Opin. Neurobiol. 22, 488–495 (2012).
Hollmann, M. & Heinemann, S. Cloned glutamate receptors. Annu. Rev. Neurosci. 17, 31–108 (1994).
Agrawal, S. G. & Evans, R. H. The primary afferent depolarizing action of kainate in the rat. Br. J. Pharmacol. 87, 345–355 (1986).
Huettner, J. E. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron 5, 255–266 (1990).
Lerma, J., Paternain, A. V., Naranjo, J. R. & Mellstrom, B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proc. Natl Acad. Sci. USA 90, 11688–11692 (1993). These authors, for the first time, recorded pharmacologically isolated kainate receptor-mediated currents from neurons derived from the CNS using a newly developed non-competitive AMPA receptor antagonist, thereby setting the stage for subsequent detection of postsynaptic kainate receptors.
Traynelis, S. F. et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 62, 405–496 (2010).
Jane, D. E., Lodge, D. & Collingridge, G. L. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology 56, 90–113 (2008).
Heckmann, M., Bufler, J., Franke, C. & Dudel, J. Kinetics of homomeric GluR6 glutamate receptor channels. Biophys. J. 71, 1743–1750 (1996).
Traynelis, S. F. & Wahl, P. Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J. Physiol. 503, 513–531 (1997).
Swanson, G. T. & Heinemann, S. F. Heterogeneity of homomeric GluR5 kainate receptor desensitization expressed in HEK293 cells. J. Physiol. 513, 639–646 (1998).
Bowie, D. & Lange, G. D. Functional stoichiometry of glutamate receptor desensitization. J. Neurosci. 22, 3392–3403 (2002).
Paternain, A. V., Cohen, A., Stern-Bach, Y. & Lerma, J. A role for extracellular Na+ in the channel gating of native and recombinant kainate receptors. J. Neurosci. 23, 8641–8648 (2003).
Wong, A. Y., Fay, A. M. & Bowie, D. External ions are coactivators of kainate receptors. J. Neurosci. 26, 5750–5755 (2006).
Plested, A. J. & Mayer, M. L. Structure and mechanism of kainate receptor modulation by anions. Neuron 53, 829–841 (2007).
Plested, A. J., Vijayan, R., Biggin, P. C. & Mayer, M. L. Molecular basis of kainate receptor modulation by sodium. Neuron 58, 720–735 (2008).
Bowie, D. Ion-dependent gating of kainate receptors. J. Physiol. 588, 67–81 (2010).
Paternain, A. V., Morales, M. & Lerma, J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron 14, 185–189 (1995).
Pinheiro, P. S. et al. Selective block of postsynaptic kainate receptors reveals their function at hippocampal mossy fiber synapses. Cereb. Cortex 17 Feb 2012 (doi:10.1093/cercor/bhs022).
Frerking, M. & Ohliger-Frerking, P. AMPA receptors and kainate receptors encode different features of afferent activity. J. Neurosci. 22, 7434–7443 (2002). This report laid experimental and theoretical groundwork for the now prevalent concept that postsynaptic AMPA and kainate receptors have distinct roles in synaptic integration as a result of their divergent gating kinetics.
Cossart, R., Esclapez, M., Hirsch, J. C., Bernard, C. & Ben-Ari, Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nature Neurosci. 1, 470–478 (1998).
Frerking, M., Malenka, R. C. & Nicoll, R. A. Synaptic activation of kainate receptors on hippocampal interneurons. Nature Neurosci. 1, 479–486 (1998).
Kidd, F. L. & Isaac, J. T. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature 400, 569–573 (1999).
Li, P. et al. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature 397, 161–164 (1999).
Petralia, R. S., Wang, Y. X. & Wenthold, R. J. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. J. Comp. Neurol. 349, 85–110 (1994).
Min, M. Y., Rusakov, D. A. & Kullmann, D. M. Activation of AMPA, kainate, and metabotropic receptors at hippocampal mossy fiber synapses: role of glutamate diffusion. Neuron 21, 561–570 (1998).
Kidd, F. L. & Isaac, J. T. Kinetics and activation of postsynaptic kainate receptors at thalamocortical synapses: role of glutamate clearance. J. Neurophysiol. 86, 1139–1148 (2001).
Darstein, M., Petralia, R. S., Swanson, G. T., Wenthold, R. J. & Heinemann, S. F. Distribution of kainate receptor subunits at hippocampal mossy fiber synapses. J. Neurosci. 23, 8013–8019 (2003).
Herb, A. et al. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron 8, 775–785 (1992).
Schiffer, H. H., Swanson, G. T. & Heinemann, S. F. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron 19, 1141–1146 (1997).
Swanson, G. T., Gereau, R. W., Green, T. & Heinemann, S. F. Identification of amino acid residues that control functional behavior in GluR5 and GluR6 kainate receptors. Neuron 19, 913–926 (1997).
Cui, C. & Mayer, M. L. Heteromeric kainate receptors formed by the coassembly of GluR5, GluR6, and GluR7. J. Neurosci. 19, 8281–8291 (1999).
Paternain, A. V., Herrera, M. T., Nieto, M. A. & Lerma, J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J. Neurosci. 20, 196–205 (2000).
Barberis, A., Sachidhanandam, S. & Mulle, C. GluR6/KA2 kainate receptors mediate slow-deactivating currents. J. Neurosci. 28, 6402–6406 (2008).
Mulle, C. et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature 392, 601–605 (1998). This was the first study to use gene-targeting to investigate neuronal kainate receptor function. The authors found that the GluK2 (GluR6) subunit was an essential constituent of postsynaptic kainate receptors in the CA3 region of hippocampus.
Contractor, A. et al. Loss of kainate receptor-mediated heterosynaptic facilitation of mossy-fiber synapses in KA2−/− mice. J. Neurosci. 23, 422–429 (2003).
Fernandes, H. B. et al. High-affinity kainate receptor subunits are necessary for ionotropic but not metabotropic signaling. Neuron 63, 818–829 (2009).
Schmitz, D., Frerking, M. & Nicoll, R. A. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron 27, 327–338 (2000).
Contractor, A., Swanson, G. T. & Heinemann, S. F. Kainate receptors are involved in short and long term plasticity at mossy fiber synapses in the hippocampus. Neuron 29, 209–216 (2001).
Pinheiro, P. S. et al. GluR7 is an essential subunit of presynaptic kainate autoreceptors at hippocampal mossy fiber synapses. Proc. Natl Acad. Sci. USA 104, 12181–12186 (2007).
Cossart, R. et al. Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron 29, 497–508 (2001).
Delaney, A. J. & Jahr, C. E. Kainate receptors differentially regulate release at two parallel fiber synapses. Neuron 36, 475–482 (2002).
Huang, Y. H., Dykes-Hoberg, M., Tanaka, K., Rothstein, J. D. & Bergles, D. E. Climbing fiber activation of EAAT4 transporters and kainate receptors in cerebellar Purkinje cells. J. Neurosci. 24, 103–111 (2004).
Rodríguez-Moreno, A. & Lerma, J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20, 1211–1218 (1998). This study introduces the concept of metabotropic signalling by kainate receptors acting as heterosynaptic modulators of GABA release in the CA1 region.
Melyan, Z., Wheal, H. V. & Lancaster, B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron 34, 107–114 (2002). These authors demonstrated that kainate receptors regulate intrinsic conductances in CA1 pyramidal neurons through G protein-mediated signalling pathways.
Melyan, Z., Lancaster, B. & Wheal, H. V. Metabotropic regulation of intrinsic excitability by synaptic activation of kainate receptors. J. Neurosci. 24, 4530–4534 (2004).
Fisahn, A., Heinemann, S. F. & McBain, C. J. The kainate receptor subunit GluR6 mediates metabotropic regulation of the slow and medium AHP currents in mouse hippocampal neurones. J. Physiol. 562, 199–203 (2005).
Ruiz, A., Sachidhanandam, S., Utvik, J. K., Coussen, F. & Mulle, C. Distinct subunits in heteromeric kainate receptors mediate ionotropic and metabotropic function at hippocampal mossy fiber synapses. J. Neurosci. 25, 11710–11718 (2005).
Coussen, F. Molecular determinants of kainate receptor trafficking. Neuroscience 158, 25–35 (2009).
Garcia, E. P. et al. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron 21, 727–739 (1998).
Bowie, D., Garcia, E. P., Marshall, J., Traynelis, S. F. & Lange, G. D. Allosteric regulation and spatial distribution of kainate receptors bound to ancillary proteins. J. Physiol. 547, 373–385 (2003).
Hirbec, H. et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron 37, 625–638 (2003).
Selak, S. et al. A role for SNAP25 in internalization of kainate receptors and synaptic plasticity. Neuron 63, 357–371 (2009).
Coussen, F. et al. Recruitment of the kainate receptor subunit glutamate receptor 6 by cadherin/catenin complexes. J. Neurosci. 22, 6426–6436 (2002).
Vivithanaporn, P., Yan, S. & Swanson, G. T. Intracellular trafficking of KA2 kainate receptors mediated by interactions with coatomer protein complex I (COPI) and 14-3-3 chaperone systems. J. Biol. Chem. 281, 15475–15484 (2006).
Salinas, G. D. et al. Actinfilin is a CUL3 substrate adaptor, linking GluR6 kainate receptor subunits to the ubiquitin-proteasome pathway. J. Biol. Chem. 281, 40164–40173 (2006).
Newpher, T. M. & Ehlers, M. D. Glutamate receptor dynamics in dendritic microdomains. Neuron 58, 472–497 (2008).
Laezza, F. et al. KRIP6: a novel BTB/kelch protein regulating function of kainate receptors. Mol. Cell. Neurosci. 34, 539–550 (2007).
Ng, D. et al. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. 7, e41 (2009).
Stöhr, H., Berger, C., Fröhlich, S. & Weber, B. H. A novel gene encoding a putative transmembrane protein with two extracellular CUB domains and a low-density lipoprotein class A module: isolation of alternatively spliced isoforms in retina and brain. Gene 286, 223–231 (2002).
Michishita, M. et al. A novel gene, Btcl1, encoding CUB and LDLa domains is expressed in restricted areas of mouse brain. Biochem. Biophys. Res. Commun. 306, 680–686 (2003).
Michishita, M. et al. Expression of Btcl2, a novel member of Btcl gene family, during development of the central nervous system. Dev. Brain. Res. 153, 135–142 (2004).
Bork, P. & Beckmann, G. The CUB domain: a widespread module in developmentally regulated proteins. J. Mol. Biol. 231, 539–545 (1993).
He, Z. & Tessier-Lavigne, M. Neuropilin is a receptor for the axonal chemorepellent semaphorin III. Cell 90, 739–751 (1997).
Kolodkin, A. L. et al. Neuropilin is a semaphorin III receptor. Cell 90, 753–762 (1997).
Gally, C., Eimer, S., Richmond, J. E. & Bessereau, J. L. A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans. Nature 431, 578–582 (2004).
Zheng, Y., Mellem, J. E., Brockie, P. J., Madsen, D. M. & Maricq, A. V. SOL-1 is a CUB-domain protein required for GLR-1 glutamate receptor function in C. elegans. Nature 427, 451–457 (2004). This study reports the first CUB domain-containing auxiliary subunit for iGluRs, SOL-1, using a forward genetic screen in C. elegans.
Zheng, Y. et al. SOL-1 is an auxiliary subunit that modulates the gating of GLR-1 glutamate receptors in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 103, 1100–1105 (2006).
Yan, D. & Tomita, S. Defined criteria for auxiliary subunits of glutamate receptors. J. Physiol. 590, 21–31 (2012).
Kim, Y. J., Bao, H., Bonanno, L., Zhang, B. & Serpe, M. Drosophila Neto is essential for clustering glutamate receptors at the neuromuscular junction. Genes Dev. 26, 974–987 (2012).
Jaskolski, F. et al. Subunit composition and alternative splicing regulate membrane delivery of kainate receptors. J. Neurosci. 24, 2506–2515 (2004).
Yan, S. et al. A C-terminal determinant of GluR6 kainate receptor trafficking. J. Neurosci. 24, 679–691 (2004).
Coussen, F. et al. Co-assembly of two GluR6 kainate receptor splice variants within a functional protein complex. Neuron 47, 555–566 (2005).
Milstein, A. D., Zhou, W., Karimzadegan, S., Bredt, D. S. & Nicoll, R. A. TARP subtypes differentially and dose-dependently control synaptic AMPA receptor gating. Neuron 55, 905–918 (2007).
Gill, M. B. et al. Cornichon-2 modulates AMPA receptor–transmembrane AMPA receptor regulatory protein assembly to dictate gating and pharmacology. J. Neurosci. 31, 6928–6938 (2011).
Kato, A. S., Gill, M. B., Yu, H., Nisenbaum, E. S. & Bredt, D. S. TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci. 33, 241–248 (2010).
Stern-Bach, Y., Russo, S., Neuman, M. & Rosenmund, C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron 21, 907–918 (1998).
Sun, Y. et al. Mechanism of glutamate receptor desensitization. Nature 417, 245–253 (2002).
Yelshansky, M. V., Sobolevsky, A. I., Jatzke, C. & Wollmuth, L. P. Block of AMPA receptor desensitization by a point mutation outside the ligand-binding domain. J. Neurosci. 24, 4728–4736 (2004).
Vivithanaporn, P., Lash, L. L., Marszalec, B. & Swanson, G. T. Critical roles for the M3–S2 transduction linker domain in kainate receptor assembly and postassembly trafficking. J. Neurosci. 27, 10423–10433 (2007).
Lerma, J. Kainate receptor physiology. Curr. Opin. Pharmacol. 6, 89–97 (2006).
Pinheiro, P. S. & Mulle, C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nature Rev. Neurosci. 9, 423–436 (2008).
Kamiya, H. & Ozawa, S. Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse. J. Physiol. 523, 653–665 (2000).
Lauri, S. E. et al. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron 39, 327–341 (2003).
Kwon, H. B. & Castillo, P. E. Role of glutamate autoreceptors at hippocampal mossy fiber synapses. Neuron 60, 1082–1094 (2008).
Rouach, N. et al. TARP γ-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nature Neurosci. 8, 1525–1533 (2005).
Bats, C., Groc, L. & Choquet, D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron 53, 719–734 (2007).
Opazo, P. et al. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67, 239–252 (2010).
Cossart, R. et al. Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron 35, 147–159 (2002).
DeVries, S. H. & Schwartz, E. A. Kainate receptors mediate synaptic transmission between cones and 'Off' bipolar cells in a mammalian retina. Nature 397, 157–160 (1999).
DeVries, S. H. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron 28, 847–856 (2000).
DeVries, S. H., Li, W. & Saszik, S. Parallel processing in two transmitter microenvironments at the cone photoreceptor synapse. Neuron 50, 735–748 (2006).
Gendrel, M., Rapti, G., Richmond, J. E. & Bessereau, J. L. A secreted complement-control-related protein ensures acetylcholine receptor clustering. Nature 461, 992–996 (2009).
Shepherd, J. D. & Huganir, R. L. The cell biology of synaptic plasticity: AMPA receptor trafficking. Ann. Rev. Cell Dev. Biol. 23, 613–643 (2007).
Anggono, V. & Huganir, R. L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 22, 461–469 (2012).
Isom, L. L., De Jongh, K. S. & Catterall, W. A. Auxiliary subunits of voltage-gated ion channels. Neuron 12, 1183–1194 (1994).
Arikkath, J. & Campbell, K. P. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 13, 298–307 (2003).
Walker, C. S. et al. Conserved SOL-1 proteins regulate ionotropic glutamate receptor desensitization. Proc. Natl Acad. Sci. USA 103, 10787–10792 (2006).
Schwenk, J. et al. Functional proteomics identify cornichon proteins as auxiliary subunits of AMPA receptors. Science 323, 1313–1319 (2009).
von Engelhardt, J. et al. CKAMP44: a brain-specific protein attenuating short-term synaptic plasticity in the dentate gyrus. Science 327, 1518–1522 (2010).
Schwenk, J. et al. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron 74, 621–633 (2012).
Shanks, N. F. et al. Differences in AMPA and kainate receptor interactomes faciliate identification of AMPA receptor auxiliary subunit GSG1L. Cell Rep. 1, 590–598 (2012).
Yamazaki, M. et al. A novel action of stargazin as an enhancer of AMPA receptor activity. Neurosci. Res. 50, 369–374 (2004).
Priel, A. et al. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J. Neurosci. 25, 2682–2686 (2005).
Tomita, S. et al. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature 435, 1052–1058 (2005).
Turetsky, D., Garringer, E. & Patneau, D. K. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J. Neurosci. 25, 7438–7448 (2005).
Menuz, K., Stroud, R. M., Nicoll, R. A. & Hays, F. A. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science 318, 815–817 (2007).
Cho, C.-H., St-Gelais, F., Zhang, W., Tomita, S. & Howe, J. R. Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents. Neuron 55, 890–904 (2007).
Soto, D. et al. Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, γ-5. Nature Neurosci. 12, 277–285 (2009).
Jackson, A. C. et al. Probing TARP modulation of AMPA receptor conductance with polyamine toxins. J. Neurosci. 31, 7511–7520 (2011).
Tomita, S. et al. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol. 161, 805–816 (2003).
Bedoukian, M. A., Weeks, A. M. & Partin, K. M. Different domains of the AMPA receptor direct stargazin-mediated trafficking and stargazin-mediated modulation of kinetics. J. Biol. Chem. 281, 23908–23921 (2006).
Chen, L. et al. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408, 936–943 (2000).
Schnell, E. et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl Acad. Sci. USA 99, 13902–13907 (2002).
Menuz, K., O'Brien, J. L., Karmizadegan, S., Bredt, D. S. & Nicoll, R. A. TARP redundancy is critical for maintaining AMPA receptor function. J. Neurosci. 28, 8740–8746 (2008).
Rebola, N., Sachidhanandam, S., Perrais, D., Cunha, R. A. & Mulle, C. Short-term plasticity of kainate receptor-mediated EPSCs induced by NMDA receptors at hippocampal mossy fiber synapses. J. Neurosci. 27, 3987–3993 (2007).
Acknowledgements
We thank A. Contractor (Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA) for contributing original data to this article and C. Mulle (University of Bordeaux II, Bordeaux, France) for sharing data in advance of its publication. This work was supported by grants R01 NS44322 and R01 NS071952 from the US National Institute of Neurological Disorders and Stroke to G.T.S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Recombinant receptors
-
Receptors produced subsequent to the introduction into cells of complementary DNA-encoding component receptor subunits.
- Desensitization
-
A decrease in a biological response in the continued presence of or due to repeated exposure of a receptor to an agonist.
- Excitatory postsynaptic currents
-
(EPSCs). Membrane currents arising from activation of excitatory (depolarizing) postsynaptic ligand-gated ion channels by a physiological stimulus, typically measured when membrane potential is held constant in voltage clamp experiments.
- Hippocampal mossy fibre
-
Axons arising from dentate gyrus granule cells that form bouton-type synapses on proximal dendrites of CA3 pyramidal neurons and filopodial synapses on interneurons in the stratum lucidum.
- Short-term potentiation
-
A predominantly presynaptic form of plasticity in which synaptic transmission is transiently enhanced, principally due to the engagement of mechanisms that enhance vesicle release probability.
- Chaperone proteins
-
Proteins that facilitate or are necessary for trafficking of cargo proteins through the secretory pathway in cells, which can include targeting receptors to their pre- or postsynaptic sites of action.
- Pertussis toxin
-
The causative agent of whooping cough, which causes the persistent activation of Gi proteins by catalysing ADP-ribosylation of the Gαi subunit.
- SNARE
-
Soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor.
- Deactivation
-
A reduction in a receptor-mediated biological response subsequent to the removal of agonist.
- Open probability
-
The probability that an ion channel protein is in the open state under a given experimental condition.
- Open time
-
The average time an ion channel dwells in a conducting state during a single activation event.
- Single-channel conductance
-
An expression of the rate of ion flow through an ion channel during a single activation event; it is derived from Ohm's Law as a proportionality constant equal to the inverse of resistance.
Rights and permissions
About this article
Cite this article
Copits, B., Swanson, G. Dancing partners at the synapse: auxiliary subunits that shape kainate receptor function. Nat Rev Neurosci 13, 675–686 (2012). https://doi.org/10.1038/nrn3335
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3335
This article is cited by
-
NETO2 promotes melanoma progression via activation of the Ca2+/CaMKII signaling pathway
Frontiers of Medicine (2023)
-
Mechanisms underlying the synaptic trafficking of the glutamate delta receptor GluD1
Molecular Psychiatry (2019)
-
Exciting Times: New Advances Towards Understanding the Regulation and Roles of Kainate Receptors
Neurochemical Research (2019)
-
Development of Cortical Pyramidal Cell and Interneuronal Dendrites: a Role for Kainate Receptor Subunits and NETO1
Molecular Neurobiology (2019)
-
NETO1 Regulates Postsynaptic Kainate Receptors in CA3 Interneurons During Circuit Maturation
Molecular Neurobiology (2019)