Key Points
-
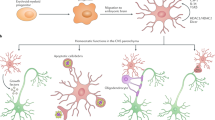
The role of infectious organisms in the aetiology of brain disease has been widely discussed. There is increasing evidence that infections do not necessarily cause neurodegenerative disease but might influence the rate of disease progression. We argue that systemic infections, or any insult that causes a systemic inflammatory response, might affect ongoing inflammation in the brain and, by further activating inflammatory cells in the brain, might exacerbate disease progression.
-
Multiple sclerosis (MS) is the archetypal inflammatory disease of the central nervous system. In the relapsing–remitting phase of the disease, systemic infections are a significant risk factor for initiating a relapse. Peripheral infection leads to cytokine synthesis in the periphery and these circulating cytokines signal across the blood–brain barrier to initiate cytokine synthesis in the brain. It seems that the inflammatory cells in the brain that are already activated or 'primed' by the pathological processes of MS are further activated by the peripheral cytokines. This leads to increased production of molecules that might be harmful to neurons.
-
In the brains of persons suffering from chronic neurodegenerative disease such as those with Alzheimer's disease (AD), the microglia are activated, as judged by their morphology and increased expression of a wide spectrum of molecules. The contribution of the activated microglia to the outcome of the disease process is not known. Systemic infections in persons in the early stages of AD can precipitate the acute onset of cognitive decline and confusion, or delirium.
-
By analogy with relapses in MS, we suggest that the activated microglia in persons with AD are primed by the pathology, and respond more vigorously than normal when the cytokines generated by the systemic infection affect the brain. Studies on animal models of chronic neurodegenerative disease show enhanced cytokine synthesis in the brain following peripheral challenge with endotoxin to mimic a systemic infection. This enhanced cytokine synthesis is associated with exaggerated behavioural symptoms.
-
Systemic infection or other insults that provoke a systemic inflammatory response can lead to a relapse in MS or to acute delirium in AD. The mechanisms underlying these acute neurological states are not fully understood. It will be important to discover whether repeated systemic infections also influence the long-term rate of decline of neurological function in these diseases.
Abstract
In multiple sclerosis — the archetypal inflammatory response in the central nervous system — T cells and macrophages invade the brain and damage the myelin and neurons. In other chronic neurodegenerative diseases, there is an atypical inflammatory response that is characterized by large numbers of activated microglia. These macrophages are primed by components of the neuropathology but might be further activated by systemic infection, which in turn has pronounced effects on inflammation in the brain and perhaps on neurological function. There is emerging evidence to support the idea that nonspecific systemic infection or inflammation in people with existing inflammation in the brain contributes to the rate of disease progression through further activation of these already primed macrophages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Anderson, D. C. & Springer, T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu. Rev. Med. 38, 175–194 (1987).
Davidson, A. & Diamond, B. Autoimmune diseases. N. Engl. J. Med. 345, 340–350 (2001).
Prat, A. et al. Migration of multiple sclerosis lymphocytes through brain endothelium. Arch. Neurol. 59, 391–397 (2002).
Ferguson, B., Matyszak, M. K., Esiri, M. M. & Perry, V. H. Axonal damage in acute multiple sclerosis lesions. Brain 120, 393–399 (1997).
Trapp, B. D. et al. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338, 278–285 (1998).
Bitsch, A., Schuchardt, J., Bunkowski, S., Kuhlmann, T. & Bruck, W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123, 1174–1183 (2000).
Brex, P. A. et al. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N. Engl. J. Med. 346, 158–164 (2002). An important study demonstrating the significant brain atrophy and neurodegeneration that is associated with MS.
Bauer, J., Rauschka, H. & Lassmann, H. Inflammation in the nervous system: the human perspective. Glia 36, 235–243 (2001).
Kivisakk, P., Trebst, C., Eckstein, D. J., Kerza-Kwiatecki, A. P. & Ransohoff, R. M. Chemokine-based therapies for MS: how do we get there from here? Trends Immunol. 22, 591–593 (2001).
Lassmann, H., Bruck, W. & Lucchinetti, C. Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy. Trends Mol. Med. 7, 115–121 (2001).
Sibley, W. A., Bamford, C. R. & Clark, K. Clinical viral infections and multiple sclerosis. Lancet 1, 1313–1315 (1985).
Panitch, H. S. Influence of infection on exacerbations of multiple sclerosis. Ann. Neurol. 36, S25–28 (1994).
Andersen, O., Lygner, P. E., Bergstrom, T., Andersson, M. & Vahlne, A. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J. Neurol. 240, 417–422 (1993).
Edwards, S., Zvartau, M., Clarke, H., Irving, W. & Blumhardt, L. D. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 64, 736–741 (1998).
Buljevac, D. et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 125, 952–960 (2002). This study demonstrates the impact of systemic infections on relapses in MS in the absence of MRI-detectable changes using gadolinium.
Jeffery, D. R. Relationship between disease activity and dose–response relationships with β interferon therapies in the treatment of multiple sclerosis. J. Neurol. Sci. 178, 2–9 (2000).
Banati, R. B. et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain 123, 2321–2337 (2000).
Newman, T. A. et al. T-cell- and macrophage-mediated axon damage in the absence of a CNS-specific immune response: involvement of metalloproteinases. Brain 124, 2203–2214 (2001).
Bickel, U., Grave, B., Kang, Y. S., del Rey, A. & Voigt, K. No increase in blood–brain barrier permeability after intraperitoneal injection of endotoxin in the rat. J. Neuroimmunol. 85, 131–136 (1998).
Smith, J. B. & Haynes, M. K. Rheumatoid arthritis — a molecular understanding. Ann. Intern. Med. 136, 908–922 (2002).
Elkayam, O., Yaron, M. & Caspi, D. Safety and efficacy of vaccination against hepatitis B in patients with rheumatoid arthritis. Ann. Rheum. Dis. 61, 623–625 (2002).
Yang, Y. X. & Lichtenstein, G. R. Corticosteroids in Crohn's disease. Am. J. Gastroenterol. 97, 803–823 (2002).
Kangro, H. O., Chong, S. K., Hardiman, A., Heath, R. B. & Walker-Smith, J. A. A prospective study of viral and mycoplasma infections in chronic inflammatory bowel disease. Gastroenterology 98, 549–553 (1990).
Beck, J. et al. Increased production of interferon γ and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol. Scand. 78, 318–323 (1988).
Palma, J. P., Park, S. H. & Kim, B. S. Treatment with lipopolysaccharide enhances the pathogenicity of a low-pathogenic variant of Theiler's murine encephalomyelitis virus. J. Neurosci. Res. 45, 776–785 (1996).
Brocke, S. et al. Induction of relapsing paralysis in experimental autoimmune encephalomyelitis by bacterial superantigen. Nature 365, 642–644 (1993).
Schiffenbauer, J., Johnson, H. M., Butfiloski, E. J., Wegrzyn, L. & Soos, J. M. Staphylococcal enterotoxins can reactivate experimental allergic encephalomyelitis. Proc. Natl Acad. Sci. USA 90, 8543–8546 (1993).
Crisi, G. M. et al. Staphylococcal enterotoxin B and tumor-necrosis factor-α-induced relapses of experimental allergic encephalomyelitis: protection by transforming growth factor-β and interleukin-10. Eur. J. Immunol. 25, 3035–3040 (1995).
Gifford, G. E. & Lohmann-Matthes, M. L. γ Interferon priming of mouse and human macrophages for induction of tumor necrosis factor production by bacterial lipopolysaccharide. J. Natl Cancer Inst. 78, 121–124 (1987).
Parant, M. A. et al. Selective modulation of lipopolysaccharide-induced death and cytokine production by various muramyl peptides. Infect. Immunol. 63, 110–115 (1995). | PubMed |
The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group. TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology 53, 457–465 (1999).
Lawson, L. J., Frost, L., Risbridger, J., Fearn, S. & Perry, V. H. Quantification of the mononuclear phagocyte response to Wallerian degeneration of the optic nerve. J. Neurocytol. 23, 729–744 (1994).
Blatteis, C. M. Role of the OVLT in the febrile response to circulating pyrogens. Prog. Brain Res. 91, 409–412 (1992).
Nguyen, M. D., Julien, J. P. & Rivest, S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nature Rev. Neurosci. 3, 216–227 (2002). A useful review on innate immunity in the CNS detailing molecular pathways that were beyond the scope of this review.
Mascarucci, P., Perego, C., Terrazzino, S. & De Simoni, M. G. Glutamate release in the nucleus tractus solitarius induced by peripheral lipopolysaccharide and interleukin-1β. Neuroscience 86, 1285–1290 (1998).
Laflamme, N., Lacroix, S. & Rivest, S. An essential role of interleukin-1β in mediating NF-κB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J. Neurosci. 19, 10923–10930 (1999).
Cao, C., Matsumura, K., Yamagata, K. & Watanabe, Y. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1β: a possible site of prostaglandin synthesis responsible for fever. Brain Res. 733, 263–272 (1996).
Ek, M. et al. Inflammatory response: pathway across the blood–brain barrier. Nature 410, 430–431 (2001).
Yamagata, K. et al. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J. Neurosci. 21, 2669–2677 (2001).
Laye, S., Parnet, P., Goujon, E. & Dantzer, R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res. Mol. Brain Res. 27, 157–162 (1994).
Konsman, J. P., Parnet, P. & Dantzer, R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 25, 154–159 (2002). This is a valuable review on the cytokine and neural pathways mediating sickness behaviour.
Stroemer, R. P. & Rothwell, N. J. Exacerbation of ischemic brain damage by localized striatal injection of interleukin-1β in the rat. J. Cereb. Blood Flow Metab. 18, 833–839 (1998).
Hardy, J. & Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Perry, V. H. & Gordon, S. Macrophages and microglia in the nervous system. Trends Neurosci. 11, 273–277 (1988).
Kreutzberg, G. W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318 (1996).
McGeer, P. L., Itagaki, S., Tago, H. & McGeer, E. G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 79, 195–200 (1987).
McGeer, P. L., Itagaki, S., Boyes, B. E. & McGeer, E. G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38, 1285–1291 (1988).
Miyazono, M. et al. A comparative immunohistochemical study of Kuru and senile plaques with a special reference to glial reactions at various stages of amyloid plaque formation. Am. J. Pathol. 139, 589–598 (1991).
Akiyama, H. et al. Inflammation and Alzheimer's disease. Neurobiol. Aging 21, 383–421 (2000).
Vitkovic, L., Bockaert, J. & Jacque, C. 'Inflammatory' cytokines: neuromodulators in normal brain? J. Neurochem. 74, 457–471 (2000).
Beard, C. M. et al. Cause of death in Alzheimer's disease. Ann. Epidemiol. 6, 195–200 (1996).
McGeer, P. L. & Rogers, J. Anti-inflammatory agents as a therapeutic approach to Alzheimer's disease. Neurology 42, 447–449 (1992).
McGeer, P. L., Schulzer, M. & McGeer, E. G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology 47, 425–432 (1996).
In 'T Veld, B. A. et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N. Engl. J. Med. 345, 1515–1521 (2001). A large prospective study demonstrating the efficacy of NSAIDs in the treatment of AD.
Aisen, P. S. Evaluation of selective COX-2 inhibitors for the treatment of Alzheimer's disease. J. Pain Symptom Manage. 23, S35–40 (2002).
Aisen, P. S., Schmeidler, J. & Pasinetti, G. M. Randomized pilot study of nimesulide treatment in Alzheimer's disease. Neurology 58, 1050–1054 (2002).
Aisen, P. S. The potential of anti-inflammatory drugs for the treatment of Alzheimer's disease. Lancet Neurol. 1, 279–284 (2002). A useful review of the present state of anti-inflammatory therapy for the treatment of AD.
Zhang, J. & Rivest, S. Anti-inflammatory effects of prostaglandin E2 in the central nervous system in response to brain injury and circulating lipopolysaccharide. J. Neurochem. 76, 855–864 (2001).
Weggen, S. et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216 (2001).
Ziegler-Heitbrock, H. W., Frankenberger, M. & Wedel, A. Tolerance to lipopolysaccharide in human blood monocytes. Immunobiology 193, 217–223 (1995).
Brown, D. R., Schmidt, B. & Kretzschmar, H. A. Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature 380, 345–347 (1996).
Meda, L. et al. Activation of microglial cells by β-amyloid protein and interferon-γ. Nature 374, 647–650 (1995).
Forloni, G. et al. Neurotoxicity of a prion protein fragment. Nature 362, 543–546 (1993).
Allen, Y. S., Devanathan, P. H. & Owen, G. P. Neurotoxicity of β-amyloid protein: cytochemical changes and apoptotic cell death investigated in organotypic cultures. Clin. Exp. Pharmacol. Physiol. 22, 370–371 (1995).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
Sturchler-Pierrat, C. et al. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl Acad. Sci. USA 94, 13287–13292 (1997).
Benzing, W. C. et al. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol. Aging 20, 581–589 (1999).
Mehlhorn, G., Hollborn, M. & Schliebs, R. Induction of cytokines in glial cells surrounding cortical β-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int. J. Dev. Neurosci. 18, 423–431 (2000).
Sly, L. M. et al. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer's disease. Brain Res. Bull. 56, 581–588 (2001). This paper demonstrates that, in an AD model, peripheral challenge with endotoxin leads to increased cytokine synthesis in the brain.
Walsh, D. T., Betmouni, S. & Perry, V. H. Absence of detectable IL-1β production in murine prion disease: a model of chronic neurodegeneration. J. Neuropathol. Exp. Neurol. 60, 173–182 (2001).
Irizarry, M. C., McNamara, M., Fedorchak, K., Hsiao, K. & Hyman, B. T. APPSw transgenic mice develop age-related Aβ deposits and neuropil abnormalities, but no neuronal loss in CA1. J. Neuropathol. Exp. Neurol. 56, 965–973 (1997).
Mucke, L. et al. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 (2000).
Lim, G. P. et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J. Neurosci. 20, 5709–5714 (2000).
Lim, G. P. et al. Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol. Aging 22, 983–991 (2001).
Jantzen, P. T. et al. Microglial activation and β-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J. Neurosci. 22, 2246–2254 (2002).
Taraboulos, A. et al. Regional mapping of prion proteins in brain. Proc. Natl Acad. Sci. USA 89, 7620–7624 (1992).
Scott, J. R., Davies, D. & Fraser, H. Scrapie in the central nervous system: neuroanatomical spread of infection and Sinc control of pathogenesis. J. Gen. Virol. 73, 1637–1644 (1992).
Gray, F. et al. Neuronal apoptosis in Creutzfeldt–Jakob disease. J. Neuropathol. Exp. Neurol. 58, 321–328 (1999).
Williams, A. E., Lawson, L. J., Perry, V. H. & Fraser, H. Characterization of the microglial response in murine scrapie. Neuropathol. Appl. Neurobiol. 20, 47–55 (1994).
Walsh, D. T., Perry, V. H. & Minghetti, L. Cyclooxygenase-2 is highly expressed in microglial-like cells in a murine model of prion disease. Glia 29, 392–396 (2000).
Cunningham, C., Boche, D. & Perry, V. H. Transforming growth factor β1, the dominant cytokine in murine prion disease: influence on inflammatory cytokine synthesis and alteration of vascular extracellular matrix. Neuropathol. Appl. Neurobiol. 28, 107–119 (2002).
Perry, V. H., Cunningham, C. & Boche, D. Atypical inflammation in the central nervous system in prion disease. Curr. Opin. Neurol. 15, 349–354 (2002).
Fadok, V. A. et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 101, 890–898 (1998).
De Simone, R., Ajmone-Cat, M. A., Nicolini, A. & Minghetti, L. Expression of phosphatidylserine receptor and down-regulation of pro-inflammatory molecule production by its natural ligand in rat microglial cultures. J. Neuropathol. Exp. Neurol. 61, 237–244 (2002).
Giese, A., Groschup, M. H., Hess, B. & Kretzschmar, H. A. Neuronal cell death in scrapie-infected mice is due to apoptosis. Brain Pathol. 5, 213–221 (1995).
Lucassen, P. J., Williams, A., Chung, W. C. & Fraser, H. Detection of apoptosis in murine scrapie. Neurosci. Lett. 198, 185–188 (1995).
Coleman, M. & Perry, V. Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci. 25, 532–537 (2002).
Guenther, K., Deacon, R. M., Perry, V. H. & Rawlins, J. N. Early behavioural changes in scrapie-affected mice and the influence of dapsone. Eur. J. Neurosci. 14, 401–409 (2001).
Betmouni, S., Perry, V. H. & Gordon, J. L. Evidence for an early inflammatory response in the central nervous system of mice with scrapie. Neuroscience 74, 1–5 (1996).
McGeer, P. L., Harada, N., Kimura, H., McGeer, E. G. & Schulzer, M. Prevalence of dementia amongst elderly Japanese with leprosy: apparent effect of chronic drug therapy. Dementia 3, 146–149 (1992).
Manuelidis, L., Fritch, W. & Zaitsev, I. Dapsone to delay symptoms in Creutzfeldt–Jakob disease. Lancet 352, 456 (1998).
Combrinck, M. I., Perry, V. H. & Cunningham, C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience 112, 7–11 (2002). A peripheral endotoxin challenge, used to mimic a systemic infection, provokes exaggerated cytokine synthesis and behavioural symptoms in animals with large numbers of activated microglia in their brains.
George, J., Bleasdale, S. & Singleton, S. J. Causes and prognosis of delirium in elderly patients admitted to a district general hospital. Age Ageing 26, 423–427 (1997).
Elie, M., Cole, M. G., Primeau, F. J. & Bellavance, F. Delirium risk factors in elderly hospitalized patients. J. Gen. Intern. Med. 13, 204–212 (1998).
Lerner, A. J., Hedera, P., Koss, E., Stuckey, J. & Friedland, R. P. Delirium in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 11, 16–20 (1997).
Rockwood, K. et al. The risk of dementia and death after delirium. Age Ageing 28, 551–556 (1999). A study highlighting the importance of acute delirium as a risk factor for dementia.
Nee, L. E. & Lippa, C. F. Alzheimer's disease in 22 twin pairs — 13-year follow-up: hormonal, infectious and traumatic factors. Dement. Geriatr. Cogn. Disord. 10, 148–151 (1999).
Murray, A. M. et al. Acute delirium and functional decline in the hospitalized elderly patient. J. Gerontol. 48, M181–186 (1993).
Engel, G. L. & Romano, J. Delirium, a syndrome of cerebral insufficiency. J. Chronic Dis. 9, 260–277 (1959).
Eikelenboom, P., Hoogendijk, W. J., Jonker, C. & van Tilburg, W. Immunological mechanisms and the spectrum of psychiatric syndromes in Alzheimer's disease. J. Psychiatr. Res. 36, 269–280 (2002).
Perry, V. H., Matyszak, M. K. & Fearn, S. Altered antigen expression of microglia in the aged rodent CNS. Glia 7, 60–67 (1993).
Sheng, J. G., Mrak, R. E. & Griffin, W. S. Enlarged and phagocytic, but not primed, interleukin-1 α-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol. (Berl.) 95, 229–234 (1998).
Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet 357, 169–175 (2001). An important community based study demonstrating that significant AD pathology is found in the brains of non-demented people living in the community. | PubMed |
Felzien, L. K., McDonald, J. T., Gleason, S. M., Berman, N. E. & Klein, R. M. Increased chemokine gene expression during aging in the murine brain. Brain Res. 890, 137–146 (2001).
Holmes, C. et al. Systemic infection, interleukin 1 and cognitive decline in Alzheimer's disease J. Neurol. Neurosurg. Psychiatry (in the press).
Schenk, D. Amyloid–β immunotherapy for Alzheimer's disease: the end of the beginning. Nature Rev. Neurosci. 3, 824–828 (2002).
Acknowledgements
The work in this laboratory is supported by the Wellcome Trust, MRC and MS Society.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Swiss-Prot
OMIM
FURTHER INFORMATION
Encyclopedia of Life Sciences
Crohn disease and ulcerative colitis
Glossary
- BLOOD–BRAIN BARRIER
-
(BBB). The physical barrier in the cerebrovasculature that restricts the movement of substances and cells between the blood and the CNS.
- CYTOKINES
-
Small proteins, secreted mostly by immune cells, that regulate the inflammatory and immune responses. These include interleukin 1β (IL1β), IL6 and tumour necrosis factor-α. The pleiotropic effects of cytokines include stimulation of cell proliferation, production of acute phase proteins, synthesis of chemokines and induction of effector enzymes.
- CHEMOKINES
-
Small proteins induced by cytokines that are involved in the chemoattraction and recruitment of subsets of leucocytes. They might also influence the activation of immune cells.
- EXPERIMENTAL ALLERGIC ENCEPHALOMYELITIS
-
(EAE). An experimental CNS inflammatory disease widely used as a model of MS. Usually induced as an acute disease in rodents in response to inoculation with spinal cord tissue homogenate or purified myelin protein peptide.
- LIPOPOLYSACCHARIDE
-
(LPS). Known also as endotoxin, this is a component of the cell wall of Gram-negative bacteria.
- TH1-TYPE CYTOKINES
-
The pattern of cytokines produced by T-helper type 1 cells, which includes large amounts of IFNγ and associated pro-inflammatory cytokines.
- STAPHYLOCOCCAL ENTEROTOXINS
-
Toxins secreted by the bacteria Staphylococcus aureus that primarily affect the intestinal system.
- SUPERANTIGEN
-
Protein that binds to and activates all T cells that express a particular set of Vβ T-cell receptor genes.
- PRIMING
-
Mechanism by which macrophages/microglia are rendered partially activated such that subsequent challenges provoke exaggerated responses.
- MURAMYL PEPTIDES
-
Fragments of peptidoglycan from the cell walls of bacteria. The immune system recognizes that muramyl peptides are bacterial products and becomes activated to resist infection.
- WALLERIAN DEGENERATION
-
The complex series of events associated with nerve fibre degeneration in the distal stump of a transected nerve.
- ACTIVATED MICROGLIA
-
Microglia that show upregulation, or de novo synthesis, of cell-surface or cytoplasmic antigens and changes in morphology such as increased numbers of processes and enlargement of the cell body. The activation of microglia by these criteria does not imply anything about their inflammatory mediator profile per se.
- ACQUIRED IMMUNITY
-
The response of antigen-specific lymphocytes to antigen and the development of immunological memory. It is mediated by the clonal selection of lymphocytes.
- INNATE IMMUNITY
-
The early response of a host to infection. One of its main features is the pro-inflammatory response induced by antigen-presenting cells — macrophages, dendritic cells and microglia.
- PROSTAGLANDINS
-
Lipophilic arachadonic acid metabolites that are synthesized by cyclooxygenase and specific prostaglandin synthases and are involved in vasodilation, pain and fever. These molecules are seen as chiefly pro-inflammatory but also have some anti-inflammatory actions.
- OPSONINS
-
Proteins or peptides that label targets for phagocytosis.
- CIRCUMVENTRICULAR ORGANS
-
(CVOs). Specialized regions of the brain that lack a patent BBB and are permeable to large molecules. They include the organum vasculosum, subfornical organ, median eminence and area postrema.
- TOLL-LIKE RECEPTORS
-
A family of receptors that are expressed at the surface of leukocytes and microglia. They are responsible for engaging the innate immune system in response to pathogens.
- VASCULAR CUFFING
-
Accumulations of lymphocytes and macrophages within the perivascular space.
- NON-STEROIDAL ANTI-INFLAMMATORY DRUGS
-
(NSAIDs). The main targets of these drugs are thought to be the prostaglandin synthetic enzymes cyclooxygenase 1 and 2, although they also affect other inflammatory mediators.
- PRESENILIN-1
-
Mutations in this protein can lead to familial AD. It is thought to be involved in the processing of APP to form Aβ.
- MAJOR HISTOCOMBATIBILITY COMPLEX CLASS II
-
There are two classes of MHC (major histocompatability complex) molecules. MHC class I molecules are found on the surface of most cells and present proteins that are generated in the cytosol to T lymphocytes. MHC class II molecules are expressed only at the surface of activated antigen-presenting cells, and they present peptides that have been degraded in cellular vesicles to T cells.
Rights and permissions
About this article
Cite this article
Perry, V., Newman, T. & Cunningham, C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci 4, 103–112 (2003). https://doi.org/10.1038/nrn1032
Issue Date:
DOI: https://doi.org/10.1038/nrn1032
This article is cited by
-
Hippocampal Microglia Activation Induced by Acute Pancreatic Injury in Rats
Digestive Diseases and Sciences (2024)
-
Effect of Heat Stress on Hippocampal Neurogenesis: Insights into the Cellular and Molecular Basis of Neuroinflammation-Induced Deficits
Cellular and Molecular Neurobiology (2023)
-
Traumatic brain injury alters dendritic cell differentiation and distribution in lymphoid and non-lymphoid organs
Journal of Neuroinflammation (2022)
-
Dendritic Cell–Targeted Therapies to Treat Neurological Disorders
Molecular Neurobiology (2022)
-
The Effects of Modified Curcumin Preparations on Glial Morphology in Aging and Neuroinflammation
Neurochemical Research (2022)