Abstract
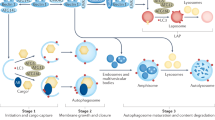
The discovery that heterozygous and homozygous mutations in the gene encoding progranulin are causally linked to frontotemporal dementia and lysosomal storage disease, respectively, reveals previously unrecognized roles of the progranulin protein in regulating lysosome biogenesis and function. Given the importance of lysosomes in cellular homeostasis, it is not surprising that progranulin deficiency has pleiotropic effects on neural circuit development and maintenance, stress response, innate immunity and ageing. This Progress article reviews recent advances in progranulin biology emphasizing its roles in lysosomal function and brain innate immunity, and outlines future avenues of investigation that may lead to new therapeutic approaches for neurodegeneration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bateman, A. & Bennett, H. P. The granulin gene family: from cancer to dementia. Bioessays 31, 1245–1254 (2009).
Baker, M. et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442, 916–919 (2006).
Cruts, M. et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442, 920–924 (2006).
Arai, T. et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611 (2006).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
DeJesus-Hernandez, M. et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 (2011).
Renton, A. E. et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 (2011).
Cruts, M., Kumar-Singh, S. & Van Broeckhoven, C. Progranulin mutations in ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Curr. Alzheimer Res. 3, 485–491 (2006).
Cenik, B., Sephton, C. F., Kutluk Cenik, B., Herz, J. & Yu, G. Progranulin: a proteolytically processed protein at the crossroads of inflammation and neurodegeneration. J. Biol. Chem. 287, 32298–32306 (2012).
Smith, K. R. et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am. J. Hum. Genet. 90, 1102–1107 (2012).
Almeida, M. R. et al. Portuguese family with the co-occurrence of frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis phenotypes due to progranulin gene mutation. Neurobiol. Aging 41, 200.e1–201.e5 (2016).
Kessenbrock, K. et al. Proteinase 3 and neutrophil elastase enhance inflammation in mice by inactivating antiinflammatory progranulin. J. Clin. Invest. 118, 2438–2447 (2008).
Salazar, N. et al. The progranulin cleavage products, granulins, exacerbate TDP-43 toxicity and increase TDP-43 levels. J. Neurosci. 35, 9315–9328 (2015).
Zhu, J. et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 111, 867–878 (2002).
Tolkatchev, D. et al. Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein Sci. 17, 711–724 (2008).
Palfree, R. G., Bennett, H. P. & Bateman, A. The evolution of the secreted regulatory protein progranulin. PLoS ONE 10, e0133749 (2015).
Lavergne, V., Taft, R. J. & Alewood, P. F. Cysteine-rich mini-proteins in human biology. Curr. Top. Med. Chem. 12, 1514–1533 (2012).
Kanazawa, M. et al. Multiple therapeutic effects of progranulin on experimental acute ischaemic stroke. Brain 138, 1932–1948 (2015).
Songsrirote, K., Li, Z., Ashford, D., Bateman, A. & Thomas-Oates, J. Development and application of mass spectrometric methods for the analysis of progranulin N-glycosylation. J. Proteomics 73, 1479–1490 (2010).
Daniel, R., Daniels, E., He, Z. & Bateman, A. Progranulin (acrogranin/PC cell-derived growth factor/granulin-epithelin precursor) is expressed in the placenta, epidermis, microvasculature, and brain during murine development. Dev. Dyn. 227, 593–599 (2003).
Daniel, R., He, Z., Carmichael, K. P., Halper, J. & Bateman, A. Cellular localization of gene expression for progranulin. J. Histochem. Cytochem. 48, 999–1009 (2000).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Petoukhov, E. et al. Activity-dependent secretion of progranulin from synapses. J. Cell Sci. 126, 5412–5421 (2013).
Lui, H. et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell 165, 921–935 (2016).
Nicholson, A. M. et al. Progranulin protein levels are differently regulated in plasma and CSF. Neurology 82, 1871–1878 (2014).
Van Damme, P. et al. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J. Cell Biol. 181, 37–41 (2008).
Wang, J. et al. Pathogenic cysteine mutations affect progranulin function and production of mature granulins. J. Neurochem. 112, 1305–1315 (2010).
Gao, X. et al. Progranulin promotes neurite outgrowth and neuronal differentiation by regulating GSK-3ß. Protein Cell 1, 552–562 (2010).
Gass, J. et al. Progranulin regulates neuronal outgrowth independent of sortilin. Mol. Neurodegener. 7, 33 (2012).
Tapia, L. et al. Progranulin deficiency decreases gross neural connectivity but enhances transmission at individual synapses. J. Neurosci. 31, 11126–11132 (2011).
Hu, F. et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 68, 654–667 (2010).
Tang, W. et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332, 478–484 (2011).
Chen, X. et al. Progranulin does not bind tumor necrosis factor (TNF) receptors and is not a direct regulator of TNF-dependent signaling or bioactivity in immune or neuronal cells. J. Neurosci. 33, 9202–9213 (2013).
De Muynck, L. et al. The neurotrophic properties of progranulin depend on the granulin E domain but do not require sortilin binding. Neurobiol. Aging 34, 2541–2547 (2013).
Neill, T. et al. EphA2 is a functional receptor for the growth factor progranulin. J. Cell Biol. 215, 687–703 (2016).
Gass, J. et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum. Mol. Genet. 15, 2988–3001 (2006).
Shankaran, S. S. et al. Missense mutations in the progranulin gene linked to frontotemporal lobar degeneration with ubiquitin-immunoreactive inclusions reduce progranulin production and secretion. J. Biol. Chem. 283, 1744–1753 (2008).
Finch, N. et al. Plasma progranulin levels predict progranulin mutation status in frontotemporal dementia patients and asymptomatic family members. Brain 132, 583–591 (2009).
Neumann, M., Kwong, L. K., Sampathu, D. M., Trojanowski, J. Q. & Lee, V. M. TDP-43 proteinopathy in frontotemporal lobar degeneration and amyotrophic lateral sclerosis: protein misfolding diseases without amyloidosis. Arch. Neurol. 64, 1388–1394 (2007).
Carcel-Trullols, J., Kovacs, A. D. & Pearce, D. A. Cell biology of the NCL proteins: what they do and don't do. Biochim. Biophys. Acta 1852, 2242–2255 (2015).
Ward, M. E. et al. Individuals with progranulin haploinsufficiency exhibit features of neuronal ceroid lipofuscinosis. Sci. Transl Med. 9, http://stm.sciencemag.org/content/9/385/eaah5642 (2017).
Gotzl, J. K. et al. Common pathobiochemical hallmarks of progranulin-associated frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis. Acta Neuropathol. 127, 845–860 (2014).
Filiano, A. J. et al. Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice. J. Neurosci. 33, 5352–5361 (2013).
Ahmed, Z. et al. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggests a role for progranulin in successful aging. Am. J. Pathol. 177, 311–324 (2010).
Martens, L. H. et al. Progranulin deficiency promotes neuroinflammation and neuron loss following toxin-induced injury. J. Clin. Invest. 122, 3955–3959 (2012).
Yin, F. et al. Exaggerated inflammation, impaired host defense, and neuropathology in progranulin-deficient mice. J. Exp. Med. 207, 117–128 (2010).
Yin, F. et al. Behavioral deficits and progressive neuropathology in progranulin-deficient mice: a mouse model of frontotemporal dementia. FASEB J 24, 4639–4647 (2010).
Almeida, S., Zhou, L. & Gao, F. B. Progranulin, a glycoprotein deficient in frontotemporal dementia, is a novel substrate of several protein disulfide isomerase family proteins. PLoS ONE 6, e26454 (2011).
Butler, G. S., Dean, R. A., Tam, E. M. & Overall, C. M. Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: dynamics of matrix metalloproteinase-14 (MT1-MMP) mediated membrane protein shedding. Mol. Cell. Biol. 28, 4896–4914 (2008).
Bai, X. H. et al. ADAMTS-7, a direct target of PTHrP, adversely regulates endochondral bone growth by associating with and inactivating GEP growth factor. Mol. Cell. Biol. 29, 4201–4219 (2009).
Suh, H. S., Choi, N., Tarassishin, L. & Lee, S. C. Regulation of progranulin expression in human microglia and proteolysis of progranulin by matrix metalloproteinase-12 (MMP-12). PLoS ONE 7, e35115 (2012).
Capell, A. et al. Rescue of progranulin deficiency associated with frontotemporal lobar degeneration by alkalizing reagents and inhibition of vacuolar ATPase. J. Neurosci. 31, 1885–1894 (2011).
Kishimoto, Y., Hiraiwa, M. & O'Brien, J. S. Saposins: structure, function, distribution, and molecular genetics. J. Lipid Res. 33, 1255–1267 (1992).
Meyer, R. C., Giddens, M. M., Coleman, B. M. & Hall, R. A. The protective role of prosaposin and its receptors in the nervous system. Brain Res. 1585, 1–12 (2014).
Lefrancois, S., Zeng, J., Hassan, A. J., Canuel, M. & Morales, C. R. The lysosomal trafficking of sphingolipid activator proteins (SAPs) is mediated by sortilin. EMBO J. 22, 6430–6437 (2003).
Hassan, A. J., Zeng, J., Ni, X. & Morales, C. R. The trafficking of prosaposin (SGP-1) and GM2AP to the lysosomes of TM4 Sertoli cells is mediated by sortilin and monomeric adaptor proteins. Mol. Reprod. Dev. 68, 476–483 (2004).
Zeng, J., Racicott, J. & Morales, C. R. The inactivation of the sortilin gene leads to a partial disruption of prosaposin trafficking to the lysosomes. Exp. Cell Res. 315, 3112–3124 (2009).
Hulkova, H. et al. A novel mutation in the coding region of the prosaposin gene leads to a complete deficiency of prosaposin and saposins, and is associated with a complex sphingolipidosis dominated by lactosylceramide accumulation. Hum. Mol. Genet. 10, 927–940 (2001).
Zhou, X. et al. Prosaposin facilitates sortilin-independent lysosomal trafficking of progranulin. J. Cell Biol. 210, 991–1002 (2015).
Nicholson, A. M. et al. Prosaposin is a regulator of progranulin levels and oligomerization. Nat. Commun. 7, 11992 (2016).
Hiesberger, T. et al. Cellular uptake of saposin (SAP) precursor and lysosomal delivery by the low density lipoprotein receptor-related protein (LRP). EMBO J. 17, 4617–4625 (1998).
Gowrishankar, S. et al. Massive accumulation of luminal protease-deficient axonal lysosomes at Alzheimer's disease amyloid plaques. Proc. Natl Acad. Sci. USA 112, E3699–E3708 (2015).
Tanaka, Y., Chambers, J. K., Matsuwaki, T., Yamanouchi, K. & Nishihara, M. Possible involvement of lysosomal dysfunction in pathological changes of the brain in aged progranulin-deficient mice. Acta Neuropathol. Commun. 2, 78 (2014).
Nixon, R. A. The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983–997 (2013).
Aharon-Peretz, J., Badarny, S., Rosenbaum, H. & Gershoni-Baruch, R. Mutations in the glucocerebrosidase gene and Parkinson disease: phenotype–genotype correlation. Neurology 65, 1460–1461 (2005).
Ramirez, A. et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat. Genet. 38, 1184–1191 (2006).
Schuur, M. et al. Cathepsin D gene and the risk of Alzheimer's disease: a population-based study and meta-analysis. Neurobiol. Aging 32, 1607–1614 (2011).
Laplante, M. & Sabatini, D. M. mTOR signaling in growth control and disease. Cell 149, 274–293 (2012).
Sardiello, M. et al. A gene network regulating lysosomal biogenesis and function. Science 325, 473–477 (2009).
Settembre, C. et al. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433 (2011).
Tanaka, Y., Matsuwaki, T., Yamanouchi, K. & Nishihara, M. Increased lysosomal biogenesis in activated microglia and exacerbated neuronal damage after traumatic brain injury in progranulin-deficient mice. Neuroscience 250, 8–19 (2013).
Belcastro, V. et al. Transcriptional gene network inference from a massive dataset elucidates transcriptome organization and gene function. Nucleic Acids Res. 39, 8677–8688 (2011).
Pickford, F. et al. Progranulin is a chemoattractant for microglia and stimulates their endocytic activity. Am. J. Pathol. 178, 284–295 (2011).
Park, B. et al. Granulin is a soluble cofactor for toll-like receptor 9 signaling. Immunity 34, 505–513 (2011).
Kao, A. W. et al. A neurodegenerative disease mutation that accelerates the clearance of apoptotic cells. Proc. Natl Acad. Sci. USA 108, 4441–4446 (2011).
Walport, M. J. Complement. First of two parts. N. Engl. J. Med. 344, 1058–1066 (2001).
Guerra, R. R., Kriazhev, L., Hernandez-Blazquez, F. J. & Bateman, A. Progranulin is a stress-response factor in fibroblasts subjected to hypoxia and acidosis. Growth Factors 25, 280–285 (2007).
Holler, C. J. et al. Trehalose upregulates progranulin expression in human and mouse models of GRN haploinsufficiency: a novel therapeutic lead to treat frontotemporal dementia. Mol. Neurodegener. 11, 46 (2016).
Cenik, R. et al. Suberoylanilide hydroxamic acid (vorinostat) up-regulates progranulin transcription: rational therapeutic approach to frontotemporal dementia. J. Biol. Chem. 286, 16101–16108 (2011).
Rademakers, R. et al. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum. Mol. Genet. 17, 3631–3642 (2008).
Hsiung, G. Y., Fok, A., Feldman, H. H., Rademakers, R. & Mackenzie, I. R. rs5848 polymorphism and serum progranulin level. J. Neurol. Sci. 300, 28–32 (2011).
Jiao, J., Herl, L. D., Farese, R. V. & Gao, F. B. MicroRNA-29b regulates the expression level of human progranulin, a secreted glycoprotein implicated in frontotemporal dementia. PLoS ONE 5, e10551 (2010).
Wang, W. X. et al. miR-107 regulates granulin/progranulin with implications for traumatic brain injury and neurodegenerative disease. Am. J. Pathol. 177, 334–345 (2010).
Liddelow, S. A. et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 (2017).
Valenzano, D. R. et al. The African turquoise killifish genome provides insights into evolution and genetic architecture of lifespan. Cell 163, 1539–1554 (2015).
Minami, S. S. et al. Progranulin protects against amyloid ß deposition and toxicity in Alzheimer's disease mouse models. Nat. Med. 20, 1157–1164 (2014).
Kelley, B. J. et al. Prominent phenotypic variability associated with mutations in Progranulin. Neurobiol. Aging 30, 739–751 (2007).
Perry, D. C. et al. Progranulin mutations as risk factors for Alzheimer disease. JAMA Neurol. 70, 774–778 (2013).
Rademakers, R. et al. Phenotypic variability associated with progranulin haploinsufficiency in patients with the common 1477C→T (Arg493X) mutation: an international initiative. Lancet Neurol. 6, 857–868 (2007).
Finn, R. D. et al. Pfam: the protein families database. Nucleic Acids Res. 42, D222–D230 (2014).
Herrero, J. et al. Ensembl comparative genomics resources. Database (Oxford) 2016, bav096 (2016).
Singh, P. P. et al. On the expansion of “dangerous” gene repertoires by whole-genome duplications in early vertebrates. Cell Rep. 2, 1387–1398 (2012).
Knopman, D. S. & Roberts, R. O. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J. Mol. Neurosci. 45, 330–335 (2011).
Mackenzie, I. R. et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 122, 111–113 (2011).
Mackenzie, I. R. et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 119, 1–4 (2010).
van Swieten, J. C. & Heutink, P. Mutations in progranulin (GRN) within the spectrum of clinical and pathological phenotypes of frontotemporal dementia. Lancet Neurol. 7, 965–974 (2008).
Acknowledgements
The authors thank W. W. Seeley, E. H. Bigio and I. R. Mackenzie for sharing the neuropathology findings in patients with frontotemporal lobar degeneration with mutations in the gene encoding progranulin. This work has been supported by US Public Health Service grants NS095257 (A.W.K.) and NS098516 (E.J.H.), the Tau Consortium (A.W.K.), the Consortium for Frontotemporal Dementia Research (E.J.H.), VA Merit Award BX002978 (E.J.H.), and the Glenn Foundation for Medical Research (A.M., P.P.S. and A.B.)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Deficiency
-
A nonspecific term used to describe both null and heterozygous loss-of-function alleles.
- Frontotemporal dementia
-
(FTD). A clinical term describing a group of disorders caused by progressive neuron loss in the frontal and/or temporal lobes of the brain. Symptoms typically manifest as personality, behaviour and language changes and can be accompanied by motor features (for additional details, see Box 2).
- Frontotemporal lobar degeneration
-
(FTLD). Umbrella term for a group of neurodegenerative conditions that affect primarily, or first, the frontal and/or temporal lobes of the brain, and that are characterized by a diverse array of neuronal inclusions comprising tau, TAR DNA-binding protein 43 or FUS.
- Granulin
-
An approximately 60 amino-acid motif characterized by highly conserved cysteines that are arranged singly or in pairs, which form six disulfide bonds.
- Haploinsufficiency
-
A loss-of-function mutation in one gene allele.
- Innate immunity
-
A relatively nonspecific part of the immune response, consists of physical barriers (such as skin and mucosa), phagocytic cells (microglia, macrophages, dendritic cells, and so on) and circulating factors (tumour necrosis factor, interferon and complement) that co-ordinately provide protection from invading pathogens, and participate in repair and maintenance of cells and organ systems.
- Lysosomal storage disease
-
A group of approximately 50 metabolic disorders that result from defective lysosomal degradation of cellular constituents, primarily affecting terminally differentiated neurons. Symptoms include seizure, blindness and developmental delay.
- Lysosome
-
A subcellular organelle found in eukaryotes containing cathepsins and other acid hydrolases that are responsible for degrading and recycling cellular constituents.
- Microglia
-
A type of non-neuronal support cells found in the CNS. Considered the resident macrophages of the brain and spinal cord, microglia are part of the innate immune system and originate from yolk sac progenitors.
- Neuronal ceroid lipofuscinosis
-
(NCL). A relatively rare subset of lysosomal storage diseases in which protein–lipid adducts known as lipofuscin accumulate in various tissues. Symptoms include blindness, epilepsy and cognitive decline.
- Nonsense mediated decay
-
A process that induces degradation of mRNAs that contain premature translation-termination codons, and constitutes an mRNA-surveillance mechanism that prevents the synthesis of truncated, potentially toxic, proteins.
- Null
-
A situation in which both alleles of a gene are mutated, leading to complete loss of gene expression.
- Overexpression
-
Excessive expression of a gene beyond the normal biologically defined levels. It can be found in pathological conditions such as cancer or it can be induced as part of experimental manipulation of a gene.
- Progranulin
-
Also known as proepithelin, granulin-epithelin precursor, acrogranin, PC cell-derived growth factor and epithelial transforming growth factor, this precursor protein has pleotropic effects in neurons owing to its effects on lysosomal function.
- Proteostasis
-
A linguistic blend of 'protein' and 'homeostasis' that refers to the cellular processes regulating the production, folding, trafficking and degradation of proteins.
- TAR DNA-binding protein 43
-
(TDP43). The protein encoded by the TARDBP gene. TDP43 acts as a transcriptional regulator that shuttles between the nucleus and the cytoplasm. In certain forms of frontotemporal lobar degeneration, including those due to mutations in the genes encoding progranulin and C9ORF72, it forms intra-cytoplasmic aggregates of hyperphosphorylated, cleaved proteins.
Rights and permissions
About this article
Cite this article
Kao, A., McKay, A., Singh, P. et al. Progranulin, lysosomal regulation and neurodegenerative disease. Nat Rev Neurosci 18, 325–333 (2017). https://doi.org/10.1038/nrn.2017.36
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2017.36
This article is cited by
-
Progranulin haploinsufficiency mediates cytoplasmic TDP-43 aggregation with lysosomal abnormalities in human microglia
Journal of Neuroinflammation (2024)
-
Reduced progranulin increases tau and α-synuclein inclusions and alters mouse tauopathy phenotypes via glucocerebrosidase
Nature Communications (2024)
-
Efferocytosis reprograms the tumor microenvironment to promote pancreatic cancer liver metastasis
Nature Cancer (2024)
-
Single-cell mapping of lipid metabolites using an infrared probe in human-derived model systems
Nature Communications (2024)
-
Progranulin Facilitates Corneal Repair Through Dual Mechanisms of Inflammation Suppression and Regeneration Promotion
Inflammation (2024)