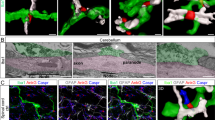
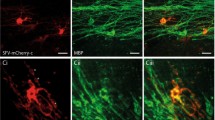
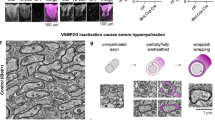
Abstract
It is widely recognized that myelination of axons greatly enhances the speed of signal transmission. An exciting new finding is the dynamic communication between axons and their myelin-forming oligodendrocytes, including activity-dependent signalling from axon to myelin. The oligodendrocyte–myelin complex may in turn respond by providing metabolic support or alter subtle myelin properties to modulate action potential propagation. In this Opinion, we discuss what is known regarding the molecular physiology of this novel, synapse-like communication and speculate on potential roles in disease states including multiple sclerosis, schizophrenia and Alzheimer disease. An emerging appreciation of the contribution of white-matter perturbations to neurological dysfunction identifies the axo-myelinic synapse as a potential novel therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
14 December 2017
In this Opinion article, the first names of the authors did not appear. The error has been corrected in the online versions of the article. The editors apologize to the authors and readers for this error.
References
Saab, A. S. et al. Oligodendroglial NMDA Receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132 (2016).
Fünfschilling, U. et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485, 517–521 (2012).
Micu, I. et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992 (2006).
Micu, I. et al. Real-time measurement of free Ca2+ changes in CNS myelin by two-photon microscopy. Nat. Med. 13, 874–879 (2007).
Stys, P. K. The axo-myelinic synapse. Trends Neurosci. 34, 393–400 (2011).
Micu, I. et al. The molecular physiology of the axo-myelinic synapse. Exp. Neurol. 276, 41–50 (2016).
Káradóttir, R., Cavelier, P., Bergersen, L. H. & Attwell, D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166 (2005).
Burzomato, V., Frugier, G., Pérez- Otaño, I., Kittler, J. T. & Attwell, D. The receptor subunits generating NMDA receptor mediated currents in oligodendrocytes. J. Physiol. 588, 3403–3414 (2010).
Nave, K.-A. Myelination and support of axonal integrity by glia. Nature 468, 244–252 (2010).
Bergles, D. E., Roberts, J. D., Somogyi, P. & Jahr, C. E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191 (2000).
Wake, H., Lee, P. R. & Fields, R. D. Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 (2011).
Schlaepfer, W. W. Vesicular disruption of myelin simulated by exposure of nerve to calcium ionophore. Nature 265, 734–736 (1977).
Káradóttir, R., Hamilton, N. B., Bakiri, Y. & Attwell, D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat. Neurosci. 11, 450–456 (2008).
Lin, S.-C. & Bergles, D. E. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 7, 24–32 (2004).
Ziskin, J. L., Nishiyama, A., Rubio, M., Fukaya, M. & Bergles, D. E. Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 10, 321–330 (2007).
Kukley, M., Capetillo-Zarate, E. & Dietrich, D. Vesicular glutamate release from axons in white matter. Nat. Neurosci. 10, 311–320 (2007).
Ge, W.-P. et al. Long-term potentiation of neuron-glia synapses mediated by Ca2+-permeable AMPA receptors. Science 312, 1533–1537 (2006).
Kang, S. H., Fukaya, M., Yang, J. K., Rothstein, J. D. & Bergles, D. E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 68, 668–681 (2010).
Rivers, L. E. et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392–1401 (2008).
Ge, W.-P., Zhou, W., Luo, Q., Jan, L. Y. & Jan, Y. N. Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc. Natl Acad. Sci. USA 106, 328–333 (2009).
Kukley, M. et al. Glial cells are born with synapses. FASEB J. 22, 2957–2969 (2008).
Fannon, J., Tarmier, W. & Fulton, D. Neuronal activity and AMPA-type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia 63, 1021–1035 (2015).
Yuan, X., Eisen, A. M., McBain, C. J. & Gallo, V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development 125, 2901–2914 (1998).
Zonouzi, M. et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci. 18, 674–682 (2015).
Gudz, T. I., Komuro, H. & Macklin, W. B. Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J. Neurosci. 26, 2458–2466 (2006).
Mangin, J.-M., Li, P., Scafidi, J. & Gallo, V. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat. Neurosci. 15, 1192–1194 (2012).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
Mensch, S. et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630 (2015).
Koudelka, S. et al. Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr. Biol. 26, 1447–1455 (2016).
De Biase, L. M. et al. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J. Neurosci. 31, 12650–12662 (2011).
Lundgaard, I. et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743 (2013).
Kougioumtzidou, E. et al. Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. eLife 6, 31 (2017).
Kukley, M., Nishiyama, A. & Dietrich, D. The fate of synaptic input to NG2 glial cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J. Neurosci. 30, 8320–8331 (2010).
Hines, J. H., Ravanelli, A. M., Schwindt, R., Scott, E. K. & Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015).
Catterall, W. A. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium 24, 307–323 (1998).
Yin, X. et al. Proteolipid protein-deficient myelin promotes axonal mitochondrial dysfunction via altered metabolic coupling. J. Cell Biol. 215, 531–542 (2016).
Ouardouz, M., Malek, S., Coderre, E. & Stys, P. K. Complex interplay between glutamate receptors and intracellular Ca2+ stores during ischaemia in rat spinal cord white matter. J. Physiol. 577, 191–204 (2006).
Ouardouz, M. et al. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann. Neurol. 65, 160–166 (2009).
Fern, R., Ransom, B. R. & Waxman, S. G. Voltage-gated calcium channels in CNS white matter: role in anoxic injury. J. Neurophysiol. 74, 369–377 (1995).
Alix, J. J. P., Dolphin, A. C. & Fern, R. Vesicular apparatus, including functional calcium channels, are present in developing rodent optic nerve axons and are required for normal node of Ranvier formation. J. Physiol. 586, 4069–4089 (2008).
Ouardouz, M. et al. Depolarization-induced Ca2+ release in ischemic spinal cord white matter involves L-type Ca2+ channel activation of ryanodine receptors. Neuron 40, 53–63 (2003).
Stirling, D. P., Cummins, K., Wayne Chen, S. R. & Stys, P. Axoplasmic reticulum Ca2+ release causes secondary degeneration of spinal axons. Ann. Neurol. 75, 220–229 (2014).
Hamilton, N. B., Kolodziejczyk, K., Kougioumtzidou, E. & Attwell, D. Proton-gated Ca2+-permeable TRP channels damage myelin in conditions mimicking ischaemia. Nature 529, 523–527 (2016).
Piña-Crespo, J. C. et al. Excitatory glycine responses of CNS myelin mediated by NR1/NR3 'NMDA' receptor subunits. J. Neurosci. 30, 11501–11505 (2010).
Micu, I., Brideau, C., Lu, L. & Stys, P. K. Effects of laser polarization on responses of the fluorescent Ca2+ indicator X-Rhod-1 in neurons and myelin. Neurophotonics 4, 025002 (2017).
Ishii, A. et al. Human myelin proteome and comparative analysis with mouse myelin. Proc. Natl Acad. Sci. USA 106, 14605–14610 (2009).
Ray, S. K. et al. Calpain inhibitor prevented apoptosis and maintained transcription of proteolipid protein and myelin basic protein genes in rat spinal cord injury. J. Chem. Neuroanat. 26, 119–124 (2003).
Wood, D. D. et al. Myelin localization of peptidylarginine deiminases 2 and 4: comparison of PAD2 and PAD4 activities. Lab. Invest. 88, 354–364 (2008).
Reiss, D. S., Lees, M. B. & Sapirstein, V. S. Is Na+ ATPase a myelin-associated enzyme? J. Neurochem. 36, 1418–1426 (1981).
Maxwell, W. L., McCreath, B. J., Graham, D. I. & Gennarelli, T. A. Cytochemical evidence for redistribution of membrane pump calcium-ATPase and ecto-Ca-ATPase activity, and calcium influx in myelinated nerve fibres of the optic nerve after stretch injury. J. Neurocytol. 24, 925–942 (1995).
Mata, M., Fink, D. J., Ernst, S. A. & Siegel, G. J. Immunocytochemical demonstration of Na+,K+-ATPase in internodal axolemma of myelinated fibers of rat sciatic and optic nerves. J. Neurochem. 57, 184–192 (1991).
Patzig, J. et al. Septin/anillin filaments scaffold central nervous system myelin to accelerate nerve conduction. eLife 5, 711 (2016).
Harris, J. J. & Attwell, D. The energetics of CNS white matter. J. Neurosci. 32, 356–371 (2012).
Micheva, K. D. et al. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. eLife 5, 3347 (2016).
Koch, K. et al. How much the eye tells the brain. Curr. Biol. 16, 1428–1434 (2006).
Trevisiol, A. et al. Monitoring ATP dynamics in electrically active white matter tracts. eLife 6, 27 (2017).
Griffiths, I. et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science 280, 1610–1613 (1998).
Lappe-Siefke, C. et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 33, 366–374 (2003).
Saab, A. S. & Nave, K.-A. Neuroscience: a mechanism for myelin injury. Nature 529, 474–475 (2016).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Czopka, T., Ffrench-Constant, C. & Lyons, D. A. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell 25, 599–609 (2013).
Yeung, M. S. Y. et al. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell 159, 766–774 (2014).
Fields, R. D. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat. Rev. Neurosci. 16, 756–767 (2015).
Bengtsson, S. L. et al. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 8, 1148–1150 (2005).
Scholz, J., Klein, M. C., Behrens, T. E. J. & Johansen-Berg, H. Training induces changes in white-matter architecture. Nat. Neurosci. 12, 1370–1371 (2009).
Luk, G., Bialystok, E., Craik, F. I. M. & Grady, C. L. Lifelong bilingualism maintains white matter integrity in older adults. J. Neurosci. 31, 16808–16813 (2011).
Jolles, D. et al. Plasticity of left perisylvian white-matter tracts is associated with individual differences in math learning. Brain Struct. Funct. 221, 1337–1351 (2016).
Emmorey, K., Allen, J. S., Bruss, J., Schenker, N. & Damasio, H. A morphometric analysis of auditory brain regions in congenitally deaf adults. Proc. Natl Acad. Sci. USA 100, 10049–10054 (2003).
Lao, Y. et al. A study of brain white matter plasticity in early blinds using tract-based spatial statistics and tract statistical analysis. Neuroreport 26, 1151–1154 (2015).
Makinodan, M., Rosen, K. M., Ito, S. & Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012).
Liu, J. et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 (2012).
Sampaio-Baptista, C. et al. Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci. 33, 19499–19503 (2013).
McKenzie, I. A. et al. Motor skill learning requires active central myelination. Science 346, 318–322 (2014).
Demerens, C. et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl Acad. Sci. USA 93, 9887–9892 (1996).
Arancibia-Cárcamo, I. L. et al. Node of Ranvier length as a potential regulator of myelinated axon conduction speed. eLife 6, 521 (2017).
Young, K. M. et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 (2013).
Ishii, A., Fyffe-Maricich, S. L., Furusho, M., Miller, R. H. & Bansal, R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J. Neurosci. 32, 8855–8864 (2012).
Jeffries, M. A. et al. ERK1/2 activation in preexisting oligodendrocytes of adult mice drives new myelin synthesis and enhanced CNS function. J. Neurosci. 36, 9186–9200 (2016).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Waggener, C. T., Dupree, J. L., Elgersma, Y. & Fuss, B. CaMKIIβ regulates oligodendrocyte maturation and CNS myelination. J. Neurosci. 33, 10453–10458 (2013).
Barnett, M. H. & Prineas, J. W. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann. Neurol. 55, 458–468 (2004).
Barnett, M. H., Parratt, J. D. E., Pollard, J. D. & Prineas, J. W. MS: is it one disease? Int. MS J. 16, 57–65 (2009).
Trapp, B. D. & Nave, K.-A. Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 31, 247–269 (2008).
Rodriguez, M. & Scheithauer, B. Ultrastructure of multiple sclerosis. Ultrastruct. Pathol. 18, 3–13 (1994).
Baranzini, S. E. et al. Pathway and network-based analysis of genome-wide association studies in multiple sclerosis. Hum. Mol. Genet. 18, 2078–2090 (2009).
Azevedo, C. J. et al. In vivo evidence of glutamate toxicity in multiple sclerosis. Ann. Neurol. 76, 269–278 (2014).
Nave, K.-A. & Ehrenreich, H. Myelination and oligodendrocyte functions in psychiatric diseases. JAMA Psychiatry 71, 582–584 (2014).
Dan, Y. & Poo, M.-M. Spike timing-dependent plasticity: from synapse to perception. Physiol. Rev. 86, 1033–1048 (2006).
Hakak, Y. et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl Acad. Sci. USA 98, 4746–4751 (2001).
Tamnes, C. K. & Agartz, I. White matter microstructure in early-onset schizophrenia: a systematic review of diffusion tensor imaging studies. J. Am. Acad. Child Adolesc. Psychiatry 55, 269–279 (2016).
Hattori, T. et al. DISC1 (disrupted-in-schizophrenia-1) regulates differentiation of oligodendrocytes. PLoS ONE 9, e88506 (2014).
Ripke, S. et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet. 45, 1150–1159 (2013).
Windrem, M. S. et al. Human iPSC glial mouse chimeras reveal glial contributions to schizophrenia. Cell Stem Cell 21, 195–208.e6 (2017).
Olmos-Serrano, J. L. et al. Down syndrome developmental brain transcriptome reveals defective oligodendrocyte differentiation and myelination. Neuron 89, 1208–1222 (2016).
Peters, A. The effects of normal aging on myelin and nerve fibers: a review. J. Neurocytol. 31, 581–593 (2002).
Stokin, G. B. et al. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science 307, 1282–1288 (2005).
Agosta, F. et al. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology 258, 853–863 (2011).
You, H. et al. Aβ neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc. Natl Acad. Sci. USA 109, 1737–1742 (2012).
Laurén, J., Gimbel, D. A., Nygaard, H. B., Gilbert, J. W. & Strittmatter, S. M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 457, 1128–1132 (2009).
Feder, N. Microperoxidase. An ultrastructural tracer of low molecular weight. J. Cell Biol. 51, 339–343 (1971).
Jang, B. et al. Myelin basic protein citrullination, a hallmark of central nervous system demyelination, assessed by novel monoclonal antibodies in prion diseases. Mol. Neurobiol. 63, 1945–1913 (2017).
Acknowledgements
Work in the authors' laboratories was supported by the Deutsche Forschungsgemeinschaft (Centre for Nanoscale Microscopy and Molecular Physiology of the Brain) and an European Research Council (ERC) Advanced Grant (to K.A.N.) and by the Multiple Sclerosis Society of Canada, Canadian Institutes of Health Research, Alberta Innovates — Health Solutions, Alberta Prion Research Institute, Canada Research Chairs and the Canada Foundation for Innovation (to P.K.S.).
Author information
Authors and Affiliations
Contributions
P. K. Stys: researching data for article, substantial contribution to discussion of content, writing, review/editing of manuscript before submission. I. Micu: researching data for article, substantial contribution to discussion of content, writing, review/editing of manuscript before submission. J. R. Plemel: researching data for article, substantial contribution to discussion of content, writing, review/editing of manuscript before submission. A. V. Caprariello: researching data for article, substantial contribution to discussion of content, writing, review/editing of manuscript before submission. K.-A. Nave: substantial contribution to discussion of content, writing, review/editing of manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Micu, I., Plemel, J., Caprariello, A. et al. Axo-myelinic neurotransmission: a novel mode of cell signalling in the central nervous system. Nat Rev Neurosci 19, 49–58 (2018). https://doi.org/10.1038/nrn.2017.128
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2017.128
This article is cited by
-
Mechanosensitive channel of large conductance enhances the mechanical stretching-induced upregulation of glycolysis and oxidative metabolism in Schwann cells
Cell Communication and Signaling (2024)
-
Oligodendrocyte–axon metabolic coupling is mediated by extracellular K+ and maintains axonal health
Nature Neuroscience (2024)
-
Deciphering Oligodendrocyte Lineages in the Human Fetal Central Nervous System Using Single-Cell RNA Sequencing
Molecular Neurobiology (2024)
-
Mechanisms of axonal support by oligodendrocyte-derived extracellular vesicles
Nature Reviews Neuroscience (2023)
-
Novel Insight into Glial Biology and Diseases
Neuroscience Bulletin (2023)