Key Points
-
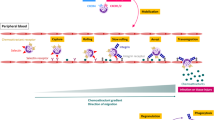
Recognition of invading bacteria is a crucial task for the human innate immune system to initiate the first line of defence against infections. For this purpose various receptors have evolved, each with different specificities for conserved microbial molecules. One important function of these receptors is to promote the migration of immune cells to the centre of infection along a gradient of microbial- or host-derived chemoattractants. This mechanism is called chemotaxis and is a crucial prerequisite for the elimination of pathogens.
-
Certain bacterial peptides can function as potent leukocyte attractants. Owing to the characteristics of bacterial translation, bacterial proteins are synthesized with a formylated methionine at the amino terminus. This feature is exploited by the human innate immune system because formylated peptides are identified as chemokines for leukocytes.
-
The chemoattractant receptor repertoire is limited and comprises members of the family of G protein-coupled receptors (GPCRs). Among them, the human formyl peptide receptor 1 (FPR1) is specialized to detect formylated peptides. Among the two orthologues of FPR1, only FPR2 has been confirmed to detect microbial peptides; FPR2 also detects endogenous ligands.
-
Recently, particular interest in FPR2 has arisen owing to the description of bacterial FPR2 ligands. These include the phenol-soluble modulin (PSM) peptides, which are cytotoxic at high concentrations and are secreted by Staphylococcus aureus and other staphylococci. FPR2 was further shown to respond differently to pathogenic and non-pathogenic staphylococcal strains according to the production of PSMs.
-
In addition to staphylococci, other bacteria such as Helicobacter pylori or enterococci activate FPR2. However, only in some instances has the nature of the agonists been identified.
-
Short-chain fatty acids are products of the energy metabolism of fermenting bacteria. They are recognized by the GPCRs GPR41 and GPR43 to mediate neutrophil chemotaxis and contribute to intestinal epithelial homeostasis.
-
Bacteria have evolved strategies to evade the host response by synthesizing molecules that block chemokine receptors. The recognition of bacterial chemotactic molecules may be beneficial for the host or, in some instances, also for pathogens taking advantage of inflammation. Moreover, chemotactic molecules are not only crucial for infection, they are also secreted by commensal bacteria and regulate epithelial homeostasis in the gut.
-
In conclusion, the field of research on microbial leukocyte chemoattractants offers interesting avenues for elucidating the complex interplay of pathogens and commensals with the immune system. Recognition of bacterial infection by the host innate immune system leads to chemotactic leukocyte influx.
Abstract
The innate immune system recognizes conserved microorganism-associated molecular patterns (MAMPs), some of which are sensed by G protein-coupled receptors (GPCRs), and this leads to chemotactic leukocyte influx. Recent studies have indicated that these processes are crucial for host defence and rely on a larger set of chemotactic MAMPs and corresponding GPCRs than was previously thought. Agonists, such as bacterial formyl peptides, enterococcal pheromone peptides, staphylococcal peptide toxins, bacterial fermentation products and the Helicobacter pylori peptide HP(2–20), stimulate specific GPCRs. The importance of leukocyte chemotaxis in host defence is highlighted by the fact that some bacterial pathogens produce chemotaxis inhibitors. How the various chemoattractants, receptors and antagonists shape antibacterial host defence represents an important topic for future research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Broz, P. & Monack, D. M. Newly described pattern recognition receptors team up against intracellular pathogens. Nature Rev. Immunol. 13, 551–565 (2013).
Park, J. & Floch, M. H. Prebiotics, probiotics, and dietary fiber in gastrointestinal disease. Gastroenterol. Clin. North Amer. 36, 47–63, v (2007).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Wentworth, C. C., Jones, R. M., Kwon, Y. M., Nusrat, A. & Neish, A. S. Commensal-epithelial signaling mediated via formyl peptide receptors. Am. J. Pathol. 177, 2782–2790 (2010).
O'Neill, L. A., Golenbock, D. & Bowie, A. G. The history of Toll-like receptors — redefining innate immunity. Nature Rev. Immunol. 13, 453–460 (2013).
Klos, A., Wende, E., Wareham, K. J. & Monk, P. N. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacol. Rev. 65, 500–543 (2013).
Honda, Z., Ishii, S. & Shimizu, T. Platelet-activating factor receptor. J. Biochem. 131, 773–779 (2002).
Di Gennaro, A. & Haeggstrom, J. Z. The leukotrienes: immune-modulating lipid mediators of disease. Adv. Immunol. 116, 51–92 (2012).
Jin, T. Gradient sensing during chemotaxis. Curr. Opin. Cell Biol. 25, 532–537 (2013).
Sun, L. & Ye, R. D. Role of G protein-coupled receptors in inflammation. Acta Pharmacol. Sin. 33, 342–350 (2012).
Rosenbaum, D. M., Rasmussen, S. G. & Kobilka, B. K. The structure and function of G-protein-coupled receptors. Nature 459, 356–363 (2009).
Venkatakrishnan, A. J. et al. Molecular signatures of G-protein-coupled receptors. Nature 494, 185–194 (2013).
Le, Y., Murphy, P. M. & Wang, J. M. Formyl-peptide receptors revisited. Trends Immunol. 23, 541–548 (2002).
Ye, R. D. et al. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161 (2009). This publication provides a comprehensive overview on FPRs and FPR agonists and antagonists.
Kretschmer, D. et al. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe 7, 463–473 (2010). This publication was the first to describe secreted bacterial FPR2 ligands and their role in S. aureus infection.
Le Poul, E. et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 (2003).
Brown, A. J. et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 (2003). This study identifies GPR41 and GPR43 as receptors for SCFAs.
Arterburn, J. B., Oprea, T. I., Prossnitz, E. R., Edwards, B. S. & Sklar, L. A. Discovery of selective probes and antagonists for G-protein-coupled receptors FPR/FPRL1 and GPR30. Curr. Top. Med. Chem. 9, 1227–1236 (2009).
Fu, H. et al. Ligand recognition and activation of formyl peptide receptors in neutrophils. J. Leukocyte Biol. 79, 247–256 (2006).
Migeotte, I., Communi, D. & Parmentier, M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 17, 501–519 (2006).
Babbin, B. A. et al. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J. Immunol. 179, 8112–8121 (2007).
Gronert, K., Gewirtz, A., Madara, J. L. & Serhan, C. N. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon γ and inhibits tumor necrosis factor α-induced IL-8 release. J. Exp. Med. 187, 1285–1294 (1998).
Tazoe, H. et al. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol. 59 (Suppl. 2), 251–262 (2008).
Riviere, S., Challet, L., Fluegge, D., Spehr, M. & Rodriguez, I. Formyl peptide receptor-like proteins are a novel family of vomeronasal chemosensors. Nature 459, 574–577 (2009).
Bao, L. et al. Mapping of genes for the human C5a receptor (C5AR), human FMLP receptor (FPR), and two FMLP receptor homologue orphan receptors (FPRH1, FPRH2) to chromosome 19. Genomics 13, 437–440 (1992).
Murphy, P. M. et al. A structural homologue of the N-formyl peptide receptor. Characterization and chromosome mapping of a peptide chemoattractant receptor family. J. Biol. Chem. 267, 7637–7643 (1992).
Sawzdargo, M. et al. A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1. Biochem. Biophys. Res. Commun. 239, 543–547 (1997).
Gao, J. L., Lee, E. J. & Murphy, P. M. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J. Exp. Med. 189, 657–662 (1999). This publication confirms a crucial role for FPR1 in bacterial infection.
Liu, M. et al. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Scientif. Rep. 2, 786 (2012).
Oldekamp, S. et al. Lack of formyl peptide receptor 1 and 2 leads to more severe inflammation and higher mortality in mice with of pneumococcal meningitis. Immunology 143, 447–461 (2014).
Zhang, Y. et al. Evaluation of human leukocyte N-formylpeptide receptor (FPR1) SNPs in aggressive periodontitis patients. Genes Immun. 4, 22–29 (2003).
Seifert, R. & Wenzel-Seifert, K. Defective Gi protein coupling in two formyl peptide receptor mutants associated with localized juvenile periodontitis. J. Biol. Chem. 276, 42043–42049 (2001).
Almkvist, J., Faldt, J., Dahlgren, C., Leffler, H. & Karlsson, A. Lipopolysaccharide-induced gelatinase granule mobilization primes neutrophils for activation by galectin-3 and formylmethionyl–Leu–Phe. Infect. Immun. 69, 832–837 (2001).
Bylund, J., Karlsson, A., Boulay, F. & Dahlgren, C. Lipopolysaccharide-induced granule mobilization and priming of the neutrophil response to Helicobacter pylori peptide HP(2–20), which activates formyl peptide receptor-like 1. Infect. Immun. 70, 2908–2914 (2002).
Bokoch, G. M. Chemoattractant signaling and leukocyte activation. Blood 86, 1649–1660 (1995).
Wenzel-Seifert, K., Arthur, J. M., Liu, H. Y. & Seifert, R. Quantitative analysis of formyl peptide receptor coupling to Giα1, Giα2, and Giα3 . J. Biol. Chem. 274, 33259–33266 (1999).
Tsu, R. C., Lai, H. W., Allen, R. A. & Wong, Y. H. Differential coupling of the formyl peptide receptor to adenylate cyclase and phospholipase C by the pertussis toxin-insensitive Gz protein. Biochem. J. 309, 331–339 (1995).
Gierschik, P., Sidiropoulos, D. & Jakobs, K. H. Two distinct Gi-proteins mediate formyl peptide receptor signal transduction in human leukemia (HL-60) cells. J. Biol. Chem. 264, 21470–21473 (1989).
Camps, M. et al. Isozyme-selective stimulation of phospholipase C-β 2 by G protein β γ-subunits. Nature 360, 684–686 (1992).
Stoyanov, B. et al. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science 269, 690–693 (1995).
Emkey, R. & Rankl, N. B. Screening G protein-coupled receptors: measurement of intracellular calcium using the fluorometric imaging plate reader. Methods Mol. Biol. 565, 145–158 (2009).
Clapham, D. E. Calcium signaling. Cell 131, 1047–1058 (2007).
Gambardella, L. & Vermeren, S. Molecular players in neutrophil chemotaxis—focus on PI3K and small GTPases. J. Leukocyte Biol. 94, 603–612 (2013).
Brahmbhatt, A. A. & Klemke, R. L. ERK and RhoA differentially regulate pseudopodia growth and retraction during chemotaxis. J. Biol. Chem. 278, 13016–13025 (2003).
Kim, D. & Haynes, C. L. The role of p38 MAPK in neutrophil functions: single cell chemotaxis and surface marker expression. Analyst 138, 6826–6833 (2013).
Ridley, A. J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Cheresh, D. A., Leng, J. & Klemke, R. L. Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells. J. Cell Biol. 146, 1107–1116 (1999).
Kikkawa, U., Matsuzaki, H. & Yamamoto, T. Protein kinase Cδ (PKCδ): activation mechanisms and functions. J. Biochem. 132, 831–839 (2002).
Babior, B. M. NADPH oxidase: an update. Blood 93, 1464–1476 (1999).
Chaves, M. M., Costa, D. C., de Oliveira, B. F., Rocha, M. I. & Nogueira-Machado, J. A. Role PKA and p38 MAPK on ROS production in neutrophil age-related: lack of IL-10 effect in older subjects. Mech. Ageing Dev. 130, 588–591 (2009).
Balazovich, K. J., Suchard, S. J., Remick, D. G. & Boxer, L. A. Tumor necrosis factor-α and FMLP receptors are functionally linked during FMLP-stimulated activation of adherent human neutrophils. Blood 88, 690–696 (1996).
Forsman, H. et al. Reactivation of desensitized formyl peptide receptors by platelet activating factor: a novel receptor cross talk mechanism regulating neutrophil superoxide anion production. PLoS ONE 8, e60169 (2013).
Reiter, E. & Lefkowitz, R. J. GRKs and β-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 17, 159–165 (2006).
Tomhave, E. D. et al. Cross-desensitization of receptors for peptide chemoattractants. Characterization of a new form of leukocyte regulation. J. Immunol. 153, 3267–3275 (1994).
Boswell, R. N., Austen, K. F. & Goetzl, E. J. A chemotactic receptor for val(ala)–gly–ser–glu on human. Immunol. Commun. 5, 469–479 (1976). This publication was the first to identify FPR1 as a chemotactic receptor.
Leeds, J. A. & Dean, C. R. Peptide deformylase as an antibacterial target: a critical assessment. Curr. Opin. Pharmacol. 6, 445–452 (2006).
Bandow, J. E. et al. The role of peptide deformylase in protein biosynthesis: a proteomic study. Proteomics 3, 299–306 (2003).
Marasco, W. A. et al. Purification and identification of formyl–methionyl–leucyl–phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J. Biol. Chem. 259, 5430–5439 (1984). This study reports the first isolation of a formylated bacterial peptide with leukocate chemoattractant activity.
Rot, A., Henderson, L. E., Copeland, T. D. & Leonard, E. J. A series of six ligands for the human formyl peptide receptor: tetrapeptides with high chemotactic potency and efficacy. Proc. Natl Acad. Sci. USA 84, 7967–7971 (1987).
Rabiet, M. J., Huet, E. & Boulay, F. Human mitochondria-derived N-formylated peptides are novel agonists equally active on FPR and FPRL1, while Listeria monocytogenes-derived peptides preferentially activate FPR. Eur. J. Immunol. 35, 2486–2495 (2005).
He, H. Q., Troksa, E. L., Caltabiano, G., Pardo, L. & Ye, R. D. Structural determinants for the interaction of formyl peptide receptor 2 with peptide ligands. J. Biol. Chem. 289, 2295–2306 (2014).
Raoof, M., Zhang, Q., Itagaki, K. & Hauser, C. J. Mitochondrial peptides are potent immune activators that activate human neutrophils via FPR-1. J. Trauma 68, 1328–1332 (2010).
Wenceslau, C. F. et al. Mitochondrial-derived N-formyl peptides: novel links between trauma, vascular collapse and sepsis. Med. Hypotheses 81, 532–535 (2013).
Gavins, F. N. et al. Leukocyte recruitment in the brain in sepsis: involvement of the annexin 1-FPR2/ALX anti-inflammatory system. FASEB J. 26, 4977–4989 (2012).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Showell, H. J. et al. The structure–activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J. Exp. Med. 143, 1154–1169 (1976).
Schiffmann, E., Corcoran, B. A. & Wahl, S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc. Natl Acad. Sci. USA 72, 1059–1062 (1975). This publication reports the discovery of synthetic formylated peptides with chemoattractant activity.
Mills, J. S. et al. Identification of a ligand binding site in the human neutrophil formyl peptide receptor using a site-specific fluorescent photoaffinity label and mass spectrometry. J. Biol. Chem. 273, 10428–10435 (1998).
Southgate, E. L. et al. Identification of formyl peptides from Listeria monocytogenes and Staphylococcus aureus as potent chemoattractants for mouse neutrophils. J. Immunol. 181, 1429–1437 (2008).
Durr, M. C. et al. Neutrophil chemotaxis by pathogen-associated molecular patterns — formylated peptides are crucial but not the sole neutrophil attractants produced by Staphylococcus aureus. Cell. Microbiol. 8, 207–217 (2006). This study demonstrates that formylated peptides are dominant bacterial chemoattractants for neutrophils.
Mader, D., Rabiet, M. J., Boulay, F. & Peschel, A. Formyl peptide receptor-mediated proinflammatory consequences of peptide deformylase inhibition in Staphylococcus aureus. Microbes Infect. 12, 415–419 (2010).
Fu, H., Dahlgren, C. & Bylund, J. Subinhibitory concentrations of the deformylase inhibitor actinonin increase bacterial release of neutrophil-activating peptides: a new approach to antimicrobial chemotherapy. Antimicrob. Agents Chemother. 47, 2545–2550 (2003).
Leibig, M. et al. Pyruvate formate lyase acts as a formate supplier for metabolic processes during anaerobiosis in Staphylococcus aureus. J. Bacteriol. 193, 952–962 (2011).
Rautenberg, M., Joo, H. S., Otto, M. & Peschel, A. Neutrophil responses to staphylococcal pathogens and commensals via the formyl peptide receptor 2 relates to phenol-soluble modulin release and virulence. FASEB J. 25, 1254–1263 (2011). This paper describes that FPR2 activation corresponds to virulence of staphylococcal strains.
Queck, S. Y. et al. Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog. 5, e1000533 (2009).
Wang, R. et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nature Med. 13, 1510–1514 (2007). This study identifies PSM peptides as important S. aureus leukotoxins and chemoattractants.
Peschel, A. & Otto, M. Phenol-soluble modulins and staphylococcal infection. Nature Rev. Microbiol. 11, 667–673 (2013). This review provides a comprehensive overview on PSM structure and function.
Chatterjee, S. S. et al. Essential Staphylococcus aureus toxin export system. Nature Med. 19, 364–367 (2013).
Forsman, H., Christenson, K., Bylund, J. & Dahlgren, C. Receptor-dependent and -independent immunomodulatory effects of phenol-soluble modulin peptides from Staphylococcus aureus on human neutrophils are abrogated through peptide inactivation by reactive oxygen species. Infect. Immun. 80, 1987–1995 (2012).
Cogen, A. L. et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J. Invest. Dermatol. 130, 192–200 (2010).
Gonzalez, D. J. et al. Novel phenol-soluble modulin derivatives in community-associated methicillin-resistant Staphylococcus aureus identified through imaging mass spectrometry. J. Biol. Chem. 287, 13889–13898 (2012).
Schreiner, J. et al. Staphylococcus aureus phenol-soluble modulin peptides modulate dendritic cell functions and increase in vitro priming of regulatory T cells. J. Immunol. 190, 3417–3426 (2013).
Nakamura, Y. et al. Staphylococcus δ-toxin induces allergic skin disease by activating mast cells. Nature 503, 397–401 (2013).
Yang, E. M., Kim, S. H., Kim, N. H. & Park, H. S. The genetic association of the FPRL1 promoter polymorphism with chronic urticaria in a Korean population. Ann. Allergy Asthma Immunol. 105, 96–97 (2010).
Kim, H. J. et al. Association analysis of formyl peptide receptor 2 (FPR2) polymorphisms and aspirin exacerbated respiratory diseases. J. Hum. Genet. 57, 247–253 (2012).
Chen, K. et al. A critical role for the G protein-coupled receptor mFPR2 in airway inflammation and immune responses. J. Immunol. 184, 3331–3335 (2010).
Arias, C. A. & Murray, B. E. The rise of the Enterococcus: beyond vancomycin resistance. Nature Rev. Microbiol. 10, 266–278 (2012).
Dunny, G. M. Enterococcal sex pheromones: signaling, social behavior, and evolution. Annu. Rev. Genet. 47, 457–482 (2013).
Ike, Y., Tanimoto, K., Tomita, H., Takeuchi, K. & Fujimoto, S. Efficient transfer of the pheromone-independent Enterococcus faecium plasmid pMG1 (Gmr) (65.1 kilobases) to Enterococcus strains during broth mating. J. Bacteriol. 180, 4886–4892 (1998).
Flannagan, S. E. & Clewell, D. B. Identification and characterization of genes encoding sex pheromone cAM373 activity in Enterococcus faecalis and Staphylococcus aureus. Mol. Microbiol. 44, 803–817 (2002).
Sannomiya, P. et al. Characterization of a class of nonformylated Enterococcus faecalis-derived neutrophil chemotactic peptides: the sex pheromones. Proc. Natl Acad. Sci. USA 87, 66–70 (1990). This study reports on the neutrophil-attractant activity of enterococcal pheromone peptides.
Ember, J. A. & Hugli, T. E. Characterization of the human neutrophil response to sex pheromones from Streptococcus faecalis. Am. J. Pathol. 134, 797–805 (1989).
Craig, P. M., Territo, M. C., Karnes, W. E. & Walsh, J. H. Helicobacter pylori secretes a chemotactic factor for monocytes and neutrophils. Gut 33, 1020–1023 (1992).
Polk, D. B. & Peek, R. M. Jr. Helicobacter pylori: gastric cancer and beyond. Nature Rev. Cancer 10, 403–414 (2010).
Mustapha, P. et al. Chemokines and antimicrobial peptides have a cag-dependent early response to Helicobacter pylori infection in primary human gastric epithelial cells. Infect. Immun. 82, 2881–2889 (2014).
Betten, A. et al. A proinflammatory peptide from Helicobacter pylori activates monocytes to induce lymphocyte dysfunction and apoptosis. J. Clin. Invest. 108, 1221–1228 (2001). This paper describes the activation of FPR2 by a H. pylori peptide.
de Paulis, A. et al. Basophils infiltrate human gastric mucosa at sites of Helicobacter pylori infection, and exhibit chemotaxis in response to H. pylori-derived peptide HP(2–20). J. Immunol. 172, 7734–7743 (2004).
Fujita, Y. et al. A novel mechanism of autolysis in Helicobacter pylori: possible involvement of peptidergic substances. Helicobacter 10, 567–576 (2005).
Phadnis, S. H. et al. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect. Immun. 64, 905–912 (1996).
Uberti, A. F. et al. Pro-inflammatory properties and neutrophil activation by Helicobacter pylori urease. Toxicon 69, 240–249 (2013).
Dundon, W. G. et al. The neutrophil-activating protein of Helicobacter pylori. Int. J. Med. Microbiol. 291, 545–550 (2002).
Hoverstad, T., Fausa, O., Bjorneklett, A. & Bohmer, T. Short-chain fatty acids in the normal human feces. Scand. J. Gastroenterol. 19, 375–381 (1984).
Hong, Y. H. et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146, 5092–5099 (2005).
Vinolo, M. A. et al. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites. Clin. Sci. 117, 331–338 (2009).
Hirasawa, A., Hara, T., Katsuma, S., Adachi, T. & Tsujimoto, G. Free fatty acid receptors and drug discovery. Biol. Pharm. Bull. 31, 1847–1851 (2008).
Cox, M. A. et al. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J. Gastroenterol. 15, 5549–5557 (2009).
Trompette, A. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature Med. 20, 159–166 (2014). This publication describes the systemic impact of SCFAs on immune functions.
Sina, C. et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 183, 7514–7522 (2009).
Cundell, D. R. et al. Inhibition of human neutrophil migration in vitro by low-molecular-mass products of nontypeable Haemophilus influenzae. Infect. Immun. 61, 2419–2424 (1993).
Grenfell, B. Boosting understanding of pertussis outbreaks. Proc. Natl Acad. Sci. USA 108, 7279–7280 (2011).
Dalpiaz, A. et al. Studies on human neutrophil biological functions by means of formyl-peptide receptor agonists and antagonists. Curr. Drug Targets Immune Endocr. Metabol. Disord. 3, 33–42 (2003).
Simon, M. I., Strathmann, M. P. & Gautam, N. Diversity of G proteins in signal transduction. Science 252, 802–808 (1991).
Howlett, A. C., Qualy, J. M. & Khachatrian, L. L. Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol. Pharmacol. 29, 307–313 (1986).
Somerville, G. A. et al. Synthesis and deformylation of Staphylococcus aureus δ-toxin are linked to tricarboxylic acid cycle activity. J. Bacteriol. 185, 6686–6694 (2003).
de Haas, C. J. et al. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 199, 687–695 (2004).
van Wamel, W. J., Rooijakkers, S. H., Ruyken, M., van Kessel, K. P. & van Strijp, J. A. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on β-hemolysin-converting bacteriophages. J. Bacteriol. 188, 1310–1315 (2006).
Haas, P. J. et al. N-terminal residues of the chemotaxis inhibitory protein of Staphylococcus aureus are essential for blocking formylated peptide receptor but not C5a receptor. J. Immunol. 173, 5704–5711 (2004).
Prat, C., Bestebroer, J., de Haas, C. J., van Strijp, J. A. & van Kessel, K. P. A new staphylococcal anti-inflammatory protein that antagonizes the formyl peptide receptor-like 1. J. Immunol. 177, 8017–8026 (2006). This study presents the FPR2 inhibitory S. aureus protein FLIPr.
Jongerius, I. et al. Staphylococcal complement evasion by various convertase-blocking molecules. J. Exp. Med. 204, 2461–2471 (2007).
Palazzolo-Ballance, A. M. et al. Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J. Immunol. 180, 500–509 (2008).
Queck, S. Y. et al. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 32, 150–158 (2008).
Stemerding, A. M. et al. Staphylococcus aureus formyl peptide receptor-like 1 inhibitor (FLIPr) and its homologue FLIPr-like are potent FcγR antagonists that inhibit IgG-mediated effector functions. J. Immunol. 191, 353–362 (2013).
Perretti, M. et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nature Med. 8, 1296–1302 (2002).
Dioszeghy, V. et al. 12/15-Lipoxygenase regulates the inflammatory response to bacterial products in vivo. J. Immunol. 181, 6514–6524 (2008).
Bhowmick, R. et al. Systemic disease during Streptococcus pneumoniae acute lung infection requires 12-lipoxygenase-dependent inflammation. J. Immunol. 191, 5115–5123 (2013).
Rooijakkers, S. H. et al. Early expression of SCIN and CHIPS drives instant immune evasion by Staphylococcus aureus. Cell. Microbiol. 8, 1282–1293 (2006).
Kretschmer, D., Nikola, N., Durr, M., Otto, M. & Peschel, A. The virulence regulator Agr controls the staphylococcal capacity to activate human neutrophils via the formyl peptide receptor 2. J. Innate Immun. 4, 201–212 (2012).
Surewaard, B. G. et al. Inactivation of staphylococcal phenol soluble modulins by serum lipoprotein particles. PLoS Pathog. 8, e1002606 (2012).
Grosz, M. et al. Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin α. Cell. Microbiol. 16, 451–465 (2014).
Surewaard, B. G. et al. Staphylococcal α-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell. Microbiol. 15, 1427–1437 (2013).
Cheng, A. G., DeDent, A. C., Schneewind, O. & Missiakas, D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 19, 225–232 (2011).
Chen, M., Zhou, H., Cheng, N., Qian, F. & Ye, R. D. Serum amyloid A1 isoforms display different efficacy at Toll-like receptor 2 and formyl peptide receptor 2. Immunobiology 219, 916–923 (2014).
Cheung, G. Y. et al. Insight into structure–function relationship in phenol-soluble modulins using an alanine screen of the phenol-soluble modulin (PSM) α3 peptide. FASEB J. 28, 153–161 (2014).
Bloes, D. A., Otto, M., Peschel, A. & Kretschmer, D. Enterococcus faecium stimulates human neutrophils via the formyl-peptide receptor 2. PLoS ONE 7, e39910 (2012).
Mellado, M., Serrano, A., Martinez, C. & Rodriguez-Frade, J. M. G protein-coupled receptor dimerization and signaling. Methods Mol. Biol. 332, 141–157 (2006).
Cooray, S. N. et al. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl Acad. Sci. USA 110, 18232–18237 (2013).
Anderson, W. F. et al. Initiation of protein synthesis in prokaryotic and eukaryotic systems. Summary of EMBO Workshop. FEBS Lett. 48, 1–6 (1974).
Adams, J. M. & Capecchi, M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc. Natl Acad. Sci. USA 55, 147–155 (1966).
Guillon, J. M., Mechulam, Y., Schmitter, J. M., Blanquet, S. & Fayat, G. Disruption of the gene for Met-tRNA(fMet) formyltransferase severely impairs growth of Escherichia coli. J. Bacteriol. 174, 4294–4301 (1992).
Margolis, P. S. et al. Peptide deformylase in Staphylococcus aureus: resistance to inhibition is mediated by mutations in the formyltransferase gene. Antimicrob. Agents Chemother. 44, 1825–1831 (2000).
Mader, D. et al. Role of N-terminal protein formylation in central metabolic processes in Staphylococcus aureus. BMC Microbiol. 13, 7 (2013).
Falconer, S. B. & Brown, E. D. New screens and targets in antibacterial drug discovery. Curr. Opin. Microbiol. 12, 497–504 (2009).
Babbin, B. A. et al. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J. Biol. Chem. 281, 19588–19599 (2006).
Bindels, L. B., Dewulf, E. M. & Delzenne, N. M. GPR43/FFA2: physiopathological relevance and therapeutic prospects. Trends Pharmacol. Sci. 34, 226–232 (2013).
Littman, D. R. & Pamer, E. G. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 10, 311–323 (2011).
Targan, S. R. & Karp, L. C. Defects in mucosal immunity leading to ulcerative colitis. Immunol. Rev. 206, 296–305 (2005).
Molloy, M. J. et al. Intraluminal containment of commensal outgrowth in the gut during infection-induced dysbiosis. Cell Host Microbe 14, 318–328 (2013).
Zeng, H. et al. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J. Immunol. 171, 3668–3674 (2003).
Neish, A. S. et al. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science 289, 1560–1563 (2000).
Leoni, G. et al. Annexin A1, formyl peptide receptor, and NOX1 orchestrate epithelial repair. J. Clin. Invest. 123, 443–454 (2013).
Suzuki, T., Yoshida, S. & Hara, H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br. J. Nutr. 100, 297–305 (2008).
Chen, K. et al. Formylpeptide receptor-2 contributes to colonic epithelial homeostasis, inflammation, and tumorigenesis. J. Clin. Invest. 123, 1694–1704 (2013). This study describes the impact of FPR2 on intestinal epithelial function.
Tang, Y., Chen, Y., Jiang, H., Robbins, G. T. & Nie, D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int. J. Cancer 128, 847–856 (2011).
Bindels, L. B. et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer 107, 1337–1344 (2012).
Cheng, T. Y. et al. Formyl peptide receptor 1 expression is associated with tumor progression and survival in gastric cancer. Anticancer Res. 34, 2223–2229 (2014).
Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012).
Acknowledgements
The authors are grateful for helpful discussions with their collaborators and colleagues. The authors' research is funded by the German Research Council grants SFB685 and PE805/5-1 to A.P. and TRR34 to A.P. and D.K., and by the Fortüne programme of the Medical Faculty Tübingen to D.K..
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Complement
-
An innate immune defence system of > 25 proteins that recognizes foreign objects and targets them for destruction or phagocytosis.
- Leukotrienes
-
A class of eicosanoids that are derived from arachidonic acid and have pro-inflammatory activities.
- Phenol-soluble modulins
-
(PSMs). Staphylococcal secreted peptides with a length of 20–44 amino acids that are potent bacterial agonists for formyl peptide receptor 2 (at nanomolar concentrations) and cytolytic toxins (at micromolar concentrations).
- Vomeronasal sensory neurons
-
An olfactory sensory sytem that detects chemical stimuli such as pheromones, which are produced during mating by many animals.
- Hydrophobic moment
-
A measure of the amphiphilicity of a protein helix.
Rights and permissions
About this article
Cite this article
Bloes, D., Kretschmer, D. & Peschel, A. Enemy attraction: bacterial agonists for leukocyte chemotaxis receptors. Nat Rev Microbiol 13, 95–104 (2015). https://doi.org/10.1038/nrmicro3390
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3390
This article is cited by
-
Acetate sensing by GPR43 alarms neutrophils and protects from severe sepsis
Communications Biology (2021)
-
Regulatory role of Gpr84 in the switch of alveolar macrophages from CD11blo to CD11bhi status during lung injury process
Mucosal Immunology (2020)
-
The TIPE Molecular Pilot That Directs Lymphocyte Migration in Health and Inflammation
Scientific Reports (2020)
-
Bacterial MgrB peptide activates chemoreceptor Fpr3 in mouse accessory olfactory system and drives avoidance behaviour
Nature Communications (2019)
-
Probiotics L. acidophilus and B. clausii Modulate Gut Microbiota in Th1- and Th2-Biased Mice to Ameliorate Salmonella Typhimurium-Induced Diarrhea
Probiotics and Antimicrobial Proteins (2019)