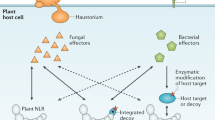
Key Points
-
Structural biology studies of proteins involved in plant pathogen–host interactions are crucial to understanding the molecular mechanisms of both pathogen virulence and host defence.
-
Plant pathogens and their hosts deploy molecules to the extracellular space (apoplast), and the structures of these molecules have revealed a relationship with membrane-damaging toxins and a mechanism for how plant receptors recognize microbial signatures.
-
Plant pathogens translocate effector proteins into host cells, and many suppress host immune defences. Structural studies of these effectors have showed how trans-kingdom protein–protein interactions have evolved to target immune regulators and have defined folds that have homology to proteins with known catalytic activities that were not apparent from protein sequence.
-
Protein structure analysis of both bacterial and oomycete effectors has identified conserved repeat folds both within and between certain effectors, which suggests evolution of protein function from a core, stable protein scaffold, probably through duplication and diversification.
-
Oligomerization of intracellular plant immune receptors (nucleotide-binding and Leu-rich repeat-containing (NB-LRR) proteins) is increasingly recognized as being important to trigger signalling in response to pathogens. Structures of the amino-terminal domains of both coiled-coil (CC) and Toll and interleukin-1 receptor (TLR) classes of NB-LRRs have shown how these regions can mediate dimerization.
Abstract
Over the past decade, considerable advances have been made in understanding the molecular mechanisms that underpin the arms race between plant pathogens and their hosts. Alongside genomic, bioinformatic, proteomic, biochemical and cell biological analyses of plant–pathogen interactions, three-dimensional structural studies of virulence proteins deployed by pathogens to promote infection, in some cases complexed with their plant cell targets, have uncovered key insights into the functions of these molecules. Structural information on plant immune receptors, which regulate the response to pathogen attack, is also starting to emerge. Structural studies of bacterial plant pathogen–host systems have been leading the way, but studies of filamentous plant pathogens are gathering pace. In this Review, we summarize the key developments in the structural biology of plant pathogen–host interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pennisi, E. Armed and dangerous. Science 327, 804–805 (2010).
Jones, N. Planetary disasters: It could happen one night. Nature 493, 154–156 (2013).
Hogenhout, S. A., Van der Hoorn, R. A., Terauchi, R. & Kamoun, S. Emerging concepts in effector biology of plant-associated organisms. Mol. Plant Microbe Interact. 22, 115–122 (2009).
Win, J. et al. Effector biology of plant-associated organisms: concepts and perspectives. Cold Spring Harb. Symp. Quant. Biol. 77, 235–247 (2012). An excellent review article summarizing key concepts that have emerged from the study of the effectors of plant-associated organisms and discussing future perspectives in the field of effector biology.
Dodds, P. N. & Rathjen, J. P. Plant immunity: towards an integrated view of plant-pathogen interactions. Nature Rev. Genet. 11, 539–548 (2010). An excellent overview of the plant immune system, with an emphasis on MAMP-triggered and effector-triggered immunity
Jones, J. D. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Thomma, B. P., Nurnberger, T. & Joosten, M. H. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell 23, 4–15 (2011).
Boller, T. & Felix, G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406 (2009).
Chisholm, S. T., Coaker, G., Day, B. & Staskawicz, B. J. Host–microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814 (2006).
Pemberton, C. L. & Salmond, G. P. The Nep1-like proteins—a growing family of microbial elicitors of plant necrosis. Mol. Plant Pathol. 5, 353–359 (2004).
Ottmann, C. et al. A common toxin fold mediates microbial attack and plant defense. Proc. Natl Acad. Sci. USA 106, 10359–10364 (2009). The three-dimensional structure of an NLP from an oomycete pathogen, which showed structural similarities between NLPs and pore-forming toxins produced by marine organisms.
Gijzen, M. & Nurnberger, T. Nep1-like proteins from plant pathogens: recruitment and diversification of the NPP1 domain across taxa. Phytochemistry 67, 1800–1807 (2006).
Qutob, D. et al. Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 18, 3721–3744 (2006).
Cabral, A. et al. Nontoxic Nep1-like proteins of the downy mildew pathogen Hyaloperonospora arabidopsidis: repression of necrosis-inducing activity by a surface-exposed region. Mol. Plant Microbe Interact. 25, 697–708 (2012).
Kanneganti, T. D., Huitema, E., Cakir, C. & Kamoun, S. Synergistic interactions of the plant cell death pathways induced by Phytophthora infestans Nepl-like protein PiNPP1.1 and INF1 elicitin. Mol. Plant Microbe Interact. 19, 854–863 (2006).
Kufner, I., Ottmann, C., Oecking, C. & Nurnberger, T. Cytolytic toxins as triggers of plant immune response. Plant Signal Behav. 4, 977–979 (2009).
Kristan, K. C., Viero, G., Dalla Serra, M., Macek, P. & Anderluh, G. Molecular mechanism of pore formation by actinoporins. Toxicon 54, 1125–1134 (2009).
Fellbrich, G. et al. NPP1, a Phytophthora-associated trigger of plant defense in parsley and Arabidopsis. Plant J. 32, 375–390 (2002).
Zaparoli, G. et al. The crystal structure of necrosis- and ethylene-inducing protein 2 from the causal agent of cacao's Witches' Broom disease reveals key elements for its activity. Biochemistry 50, 9901–9910 (2011).
Kamoun, S. A catalogue of the effector secretome of plant pathogenic oomycetes. Annu. Rev. Phytopathol. 44, 41–60 (2006).
Federici, L., Di Matteo, A., Fernandez-Recio, J., Tsernoglou, D. & Cervone, F. Polygalacturonase inhibiting proteins: players in plant innate immunity? Trends Plant Sci. 11, 65–70 (2006).
De Lorenzo, G., D'Ovidio, R. & Cervone, F. The role of polygalacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu. Rev. Phytopathol. 39, 313–335 (2001).
Brutus, A., Sicilia, F., Macone, A., Cervone, F. & De Lorenzo, G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl Acad. Sci. USA 107, 9452–9457 (2010).
Di Matteo, A. et al. The crystal structure of polygalacturonase-inhibiting protein (PGIP), a leucine-rich repeat protein involved in plant defense. Proc. Natl Acad. Sci. USA 100, 10124–10128 (2003). This article shows the crystal structure of PGIP2 from the common bean. This was the first structure of a plant LRR protein and remains one of the few cases in which the crystallized protein was purified from plants.
Casasoli, M. et al. Integration of evolutionary and desolvation energy analysis identifies functional sites in a plant immunity protein. Proc. Natl Acad. Sci. USA 106, 7666–7671 (2009).
Leckie, F. et al. The specificity of polygalacturonase-inhibiting protein (PGIP): a single amino acid substitution in the solvent-exposed β-strand/β-turn region of the leucine-rich repeats (LRRs) confers a new recognition capability. EMBO J. 18, 2352–2363 (1999).
Benedetti, M. et al. Structural resolution of the complex between a fungal polygalacturonase and a plant polygalacturonase-inhibiting protein by small-angle X-ray scattering. Plant Physiol. 157, 599–607 (2011).
Liu, T. et al. Chitin-induced dimerization activates a plant immune receptor. Science 336, 1160–1164 (2012). This article shows the crystal structure of the extracellular LysM domains of A. thaliana CERK1 in both ligand-free and chitin-bound forms, which provides evidence for ligand-induced dimerization of CERK1. The paper assesses the relevance of this dimerization for signal transduction.
Willmann, R. & Nurnberger, T. How plant lysin motif receptors get activated: Lessons learned from structural biology. Sci. Signal. 5, e28 (2012).
Miya, A. et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc. Natl Acad. Sci. USA 104, 19613–19618 (2007).
Wan, J. et al. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20, 471–481 (2008).
Petutschnig, E. K., Jones, A. M., Serazetdinova, L., Lipka, U. & Lipka, V. The lysin motif receptor-like kinase (LysM-RLK) CERK1 is a major chitin-binding protein in Arabidopsis thaliana and subject to chitin-induced phosphorylation. J. Biol. Chem. 285, 28902–28911 (2010).
Iizasa, E., Mitsutomi, M. & Nagano, Y. Direct binding of a plant LysM receptor-like kinase, LysM RLK1/CERK1, to chitin in vitro. J. Biol. Chem. 285, 2996–3004 (2010).
Hamel, L. P. & Beaudoin, N. Chitooligosaccharide sensing and downstream signaling: contrasted outcomes in pathogenic and beneficial plant–microbe interactions. Planta 232, 787–806 (2010).
Heese, A. et al. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc. Natl Acad. Sci. USA 104, 12217–12222 (2007).
Chinchilla, D. et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448, 497–500 (2007).
Nimchuk, Z. et al. Eukaryotic fatty acylation drives plasma membrane targeting and enhances function of several type III effector proteins from Pseudomonas syringae. Cell 101, 353–363 (2000).
Szurek, B., Marois, E., Bonas, U. & Van den Ackerveken, G. Eukaryotic features of the Xanthomonas type III effector AvrBs3: protein domains involved in transcriptional activation and the interaction with nuclear import receptors from pepper. Plant J. 26, 523–534 (2001).
Caillaud, M. C. et al. Subcellular localization of the Hpa RxLR effector repertoire identifies a tonoplast-associated protein HaRxL17 that confers enhanced plant susceptibility. Plant J. 69, 252–265 (2012).
Mansfield, J. W. From bacterial avirulence genes to effector functions via the hrp delivery system: an overview of 25 years of progress in our understanding of plant innate immunity. Mol. Plant Pathol. 10, 721–734 (2009).
Cheng, W. et al. Structural analysis of Pseudomonas syringae AvrPtoB bound to host BAK1 reveals two similar kinase-interacting domains in a type III effector. Cell Host Microbe 10, 616–626 (2011). This paper provides the structural characterization of the second kinase-binding domain of AvrPtoB. This domain forms a four-helix bundle that is structurally related to the first AvrProB kinase-binding domain but binds host kinases in a different orientation.
Gimenez-Ibanez, S. et al. AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr. Biol. 19, 423–429 (2009). This work shows that AvrPtoB interferes with BAK1-independent immune pathways. The first helix-bundle domain of AvrPtoB specifically binds the intracellular kinase domain of A. thaliana CERK1.
Xiao, F. et al. The N-terminal region of Pseudomonas type III effector AvrPtoB elicits Pto-dependent immunity and has two distinct virulence determinants. Plant J. 52, 595–614 (2007).
Zeng, L., Velasquez, A. C., Munkvold, K. R., Zhang, J. & Martin, G. B. A tomato LysM receptor-like kinase promotes immunity and its kinase activity is inhibited by AvrPtoB. Plant J. 69, 92–103 (2012). This is a follow-up of the structural characterization of the two AvrPtoB kinase-binding domains, which identifies a tomato RLK that is specifically inhibited by the first four-helix bundle domain of AvrPtoB.
Shan, L. et al. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 4, 17–27 (2008).
He, P. et al. Specific bacterial suppressors of MAMP signaling upstream of MAPKKK in Arabidopsis innate immunity. Cell 125, 563–575 (2006).
Janjusevic, R., Abramovitch, R. B., Martin, G. B. & Stebbins, C. E. A bacterial inhibitor of host programmed cell death defenses is an E3 ubiquitin ligase. Science 311, 222–226 (2006). This is a structure determination of a previously uncharacterized C-terminal domain of AvrPtoB, which shows that it adopts the fold of eukaryotic U-box ubiquitin E3 ligases. E3 ligase activity is required for both effector-triggered susceptibility and interference with NB-LRR-mediated immunity.
Dong, J. et al. Crystal structure of the complex between Pseudomonas effector AvrPtoB and the tomato Pto kinase reveals both a shared and a unique interface compared with AvrPto-Pto. Plant Cell 21, 1846–1859 (2009). This article shows the crystal structure of a complex between the first four-helix bundle of AvrPtoB and Pto, which reveals contact with the P + 1 loop of the kinase.
Singer, A. U. et al. Structural analysis of HopPmaL reveals the presence of a second adaptor domain common to the HopAB family of Pseudomonas syringae type III effectors. Biochemistry 51, 1–3 (2012).
Xiang, T. et al. Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr. Biol. 18, 74–80 (2008).
Xing, W. et al. The structural basis for activation of plant immunity by bacterial effector protein AvrPto. Nature 449, 243–247 (2007). This is one of the first structural characterizations of a plant pathogen effector in complex with a host protein, which establishes that AvrPto makes specific contact with the P + 1 loop of Pto and inhibits its kinase activity.
Xiang, T. et al. BAK1 is not a target of the Pseudomonas syringae effector AvrPto. Mol. Plant Microbe Interact. 24, 100–107 (2011).
Wulf, J., Pascuzzi, P. E., Fahmy, A., Martin, G. B. & Nicholson, L. K. The solution structure of type III effector protein AvrPto reveals conformational and dynamic features important for plant pathogenesis. Structure 12, 1257–1268 (2004).
Fu, Z. Q. et al. A type III effector ADP-ribosylates RNA-binding proteins and quells plant immunity. Nature 447, 284–288 (2007).
Wirthmueller, L. & Banfield, M. J. mADP-RTs: versatile virulence factors from bacterial pathogens of plants and mammals. Front. Plant Sci. 3, 142 (2012).
Nicaise, V. et al. Pseudomonas HopU1 modulates plant immune receptor levels by blocking the interaction of their mRNAs with GRP7. EMBO J. 32, 701–712 (2013).
Jeong, B. R. et al. Structure function analysis of an ADP-ribosyltransferase type III effector and its RNA-binding target in plant immunity. J. Biol. Chem. 286, 43272–43281 (2011). This paper describes the structural basis for HopU1-mediated mADP-ribosylation of the RNA-binding protein GRP7 on a specific Arg residue that is essential for RNA binding.
Schoning, J. C. et al. Auto-regulation of the circadian slave oscillator component AtGRP7 and regulation of its targets is impaired by a single RNA recognition motif point mutation. Plant J. 52, 1119–1130 (2007).
Singer, A. U. et al. Crystal structures of the type III effector protein AvrPphF and its chaperone reveal residues required for plant pathogenesis. Structure 12, 1669–1681 (2004). This is the first report of a crystal structure of an effector protein from a plant pathogen.
Wang, Y. et al. A Pseudomonas syringae ADP-ribosyltransferase inhibits Arabidopsis mitogen-activated protein kinase kinases. Plant Cell 22, 2033–2044 (2010).
Wilton, M. et al. The type III effector HopF2Pto targets Arabidopsis RIN4 protein to promote Pseudomonas syringae virulence. Proc. Natl Acad. Sci. USA 107, 2349–2354 (2010).
Gohre, V. et al. Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr. Biol. 18, 1824–1832 (2008).
Zhang, J. et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7, 290–301 (2010).
Zhu, M., Shao, F., Innes, R. W., Dixon, J. E. & Xu, Z. The crystal structure of Pseudomonas avirulence protein AvrPphB: a papain-like fold with a distinct substrate-binding site. Proc. Natl Acad. Sci. USA 101, 302–307 (2004).
Shao, F., Merritt, P. M., Bao, Z., Innes, R. W. & Dixon, J. E. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109, 575–588 (2002).
Lu, D. et al. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc. Natl Acad. Sci. USA 107, 496–501 (2010).
Ade, J., DeYoung, B. J., Golstein, C. & Innes, R. W. Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Proc. Natl Acad. Sci. USA 104, 2531–2536 (2007).
DeYoung, B. J., Qi, D., Kim, S. H., Burke, T. P. & Innes, R. W. Activation of a plant nucleotide binding-leucine rich repeat disease resistance protein by a modified self protein. Cell. Microbiol. 14, 1071–1084 (2012).
Shao, F. et al. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 301, 1230–1233 (2003).
Singer, A. U. et al. A pathogen type III effector with a novel E3 ubiquitin ligase architecture. PLoS Pathog. 9, e1003121 (2013). This is an excellent example of how structural biology can promote elucidation of the molecular functions of plant pathogen effectors.
Singer, A. U. et al. Structure of the Shigella T3SS effector IpaH defines a new class of E3 ubiquitin ligases. Nature Struct. Mol. Biol. 15, 1293–1301 (2008).
Quezada, C. M., Hicks, S. W., Galan, J. E. & Stebbins, C. E. A family of Salmonella virulence factors functions as a distinct class of autoregulated E3 ubiquitin ligases. Proc. Natl Acad. Sci. USA 106, 4864–4869 (2009).
Kim, J. G. et al. XopD SUMO protease affects host transcription, promotes pathogen growth, and delays symptom development in Xanthomonas-infected tomato leaves. Plant Cell 20, 1915–1929 (2008).
Chosed, R. et al. Structural analysis of Xanthomonas XopD provides insights into substrate specificity of ubiquitin-like protein proteases. J. Biol. Chem. 282, 6773–6782 (2007).
Kim, J. G., Stork, W. & Mudgett, M. B. Xanthomonas type III effector XopD sesumoylates tomato transcription factor SlERF4 to suppress ethylene responses and promote pathogen growth. Cell Host Microbe 13, 143–154 (2013).
Grant, S. R., Fisher, E. J., Chang, J. H., Mole, B. M. & Dangl, J. L. Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu. Rev. Microbiol. 60, 425–449 (2006).
Kim, M. G. et al. Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defense in Arabidopsis. Cell 121, 749–759 (2005).
Liu, J. et al. RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol. 7, e1000139 (2009).
Afzal, A. J., da Cunha, L. & Mackey, D. Separable fragments and membrane tethering of Arabidopsis RIN4 regulate its suppression of PAMP-triggered immunity. Plant Cell 23, 3798–3811 (2011).
Luo, Y., Caldwell, K. S., Wroblewski, T., Wright, M. E. & Michelmore, R. W. Proteolysis of a negative regulator of innate immunity is dependent on resistance genes in tomato and Nicotiana benthamiana and induced by multiple bacterial effectors. Plant Cell 21, 2458–2472 (2009).
Wilton, M. et al. The type III effector HopF2Pto targets Arabidopsis RIN4 protein to promote Pseudomonas syringae virulence. Proc. Natl Acad. Sci. USA 107, 2349–2354 (2010).
Mackey, D., Holt, B. F., 3rd, Wiig, A. & Dangl, J. L. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 108, 743–754 (2002).
Axtell, M. J., Chisholm, S. T., Dahlbeck, D. & Staskawicz, B. J. Genetic and molecular evidence that the Pseudomonas syringae type III effector protein AvrRpt2 is a cysteine protease. Mol. Microbiol. 49, 1537–1546 (2003).
Axtell, M. J. & Staskawicz, B. J. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112, 369–377 (2003).
Mackey, D., Belkhadir, Y., Alonso, J. M., Ecker, J. R. & Dangl, J. L. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112, 379–389 (2003).
Gao, Z., Chung, E. H., Eitas, T. K. & Dangl, J. L. Plant intracellular innate immune receptor resistance to Pseudomonas syringae pv. maculicola 1 (RPM1) is activated at, and functions on, the plasma membrane. Proc. Natl Acad. Sci. USA 108, 7619–7624 (2011).
Liu, J., Elmore, J. M., Lin, Z. J. & Coaker, G. A receptor-like cytoplasmic kinase phosphorylates the host target RIN4, leading to the activation of a plant innate immune receptor. Cell Host Microbe 9, 137–146 (2011).
Lee, C. C. et al. Crystal structure of the type III effector AvrB from Pseudomonas syringae. Structure 12, 487–494 (2004).
Desveaux, D. et al. Type III effector activation via nucleotide binding, phosphorylation, and host target interaction. PLoS Pathog. 3, e48 (2007). This is the first structural characterization of a plant pathogen effector (AvrB) in complex with the interacting domain of its corresponding plant protein (RIN4).
Kim, H. S. et al. The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation. Proc. Natl Acad. Sci. USA 102, 6496–6501 (2005).
Feng, F. et al. A Xanthomonas uridine 5′-monophosphate transferase inhibits plant immune kinases. Nature 485, 114–118 (2012).
Kinch, L. N., Yarbrough, M. L., Orth, K. & Grishin, N. V. Fido, a novel AMPylation domain common to fic, doc, and AvrB. PLoS ONE 4, e5818 (2009). This article reports structural similarity between AvrB, and Fic and doc proteins.
Worby, C. A. et al. The fic domain: regulation of cell signaling by adenylylation. Mol. Cell 34, 93–103 (2009).
Yarbrough, M. L. et al. AMPylation of Rho GTPases by Vibrio VopS disrupts effector binding and downstream signaling. Science 323, 269–272 (2009).
Kim, Y. J., Lin, N. C. & Martin, G. B. Two distinct Pseudomonas effector proteins interact with the Pto kinase and activate plant immunity. Cell 109, 589–598 (2002).
Ntoukakis, V. et al. The tomato Prf complex is a molecular trap for bacterial effectors based on pto transphosphorylation. PLoS Pathog. 9, e1003123 (2013).
Rosebrock, T. R. et al. A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature 448, 370–374 (2007).
Ntoukakis, V. et al. Host inhibition of a bacterial virulence effector triggers immunity to infection. Science 324, 784–787 (2009).
Zhou, J. M. & Chai, J. Plant pathogenic bacterial type III effectors subdue host responses. Curr. Opin. Microbiol. 11, 179–185 (2008).
van der Hoorn, R. A. & Kamoun, S. From guard to decoy: a new model for perception of plant pathogen effectors. Plant Cell 20, 2009–2017 (2008).
Birker, D. et al. A locus conferring resistance to Colletotrichum higginsianum is shared by four geographically distinct Arabidopsis accessions. Plant J. 60, 602–613 (2009).
Narusaka, M. et al. Interfamily transfer of dual NB-LRR genes confers resistance to multiple pathogens. PLoS ONE 8, e55954 (2013).
Narusaka, M. et al. RRS1 and RPS4 provide a dual resistance-gene system against fungal and bacterial pathogens. Plant J. 60, 218–226 (2009).
Sohn, K. H., Hughes, R. K., Piquerez, S. J., Jones, J. D. & Banfield, M. J. Distinct regions of the Pseudomonas syringae coiled-coil effector AvrRps4 are required for activation of immunity. Proc. Natl Acad. Sci. USA 109, 16371–16376 (2012). In this paper, the crystal structure of AvrRps4 shows that an electronegative surface patch in the structure is important for recognition by the NB-LRR proteins RPS4 and RRS1.
Sohn, K. H., Zhang, Y. & Jones, J. D. The Pseudomonas syringae effector protein, AvrRPS4, requires in planta processing and the KRVY domain to function. Plant J. 57, 1079–1091 (2009).
Bhattacharjee, S., Halane, M. K., Kim, S. H. & Gassmann, W. Pathogen effectors target Arabidopsis EDS1 and alter its interactions with immune regulators. Science 334, 1405–1408 (2011).
Heidrich, K. et al. Arabidopsis EDS1 connects pathogen effector recognition to cell compartment-specific immune responses. Science 334, 1401–1404 (2011).
Boch, J. & Bonas, U. Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu. Rev. Phytopathol. 48, 419–436 (2010).
Kay, S., Hahn, S., Marois, E., Hause, G. & Bonas, U. A. Bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science 318, 648–651 (2007). This work shows that TAL effectors are transcription factors and that they promote disease by directly binding to specific host gene promoters.
Romer, P. et al. Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science 318, 645–648 (2007). This article describes the molecular basis of TAL effector recognition by plants.
Boch, J. et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326, 1509–1512 (2009).
Moscou, M. J. & Bogdanove, A. J. A simple cipher governs DNA recognition by TAL effectors. Science 326, 1501 (2009). Together with reference 111, this work explains how the repeat architecture of TAL effectors determines their sequence-specific DNA-binding properties.
Bogdanove, A. J. & Voytas, D. F. TAL effectors: customizable proteins for DNA targeting. Science 333, 1843–1846 (2011).
Morbitzer, R., Römer, P., Boch, J. & Lahaye, T. Regulation of selected genome loci using de novo-engineered transcription activator-like effector (TALE)-type transcription factors. Proc. Natl Acad. Sci. USA 107, 21617–21622 (2010).
Murakami, M. T. et al. The repeat domain of the type III effector protein PthA shows a TPR-like structure and undergoes conformational changes upon DNA interaction. Proteins 78, 3386–3395 (2010).
Deng, D. et al. Structural basis for sequence-specific recognition of DNA by TAL effectors. Science 335, 720–723 (2012).
Mak, A. N., Bradley, P., Bogdanove, A. J. & Stoddard, B. L. TAL effectors: function, structure, engineering and applications. Curr. Opin. Struct. Biol. 23, 93–99 (2013).
Mak, A. N.-S., Bradley, P., Cernadas, R. A., Bogdanove, A. J. & Stoddard, B. L. The crystal structure of TAL effector PthXo1 bound to its DNA Target. Science 335, 716–719 (2012). Together with reference 116, this paper provides the structural basis for the DNA sequence specificity of TAL effectors and for the rational design of TAL proteins.
Yin, P. et al. Specific DNA–RNA hybrid recognition by TAL effectors. Cell Rep. 2, 707–713 (2012).
Deng, D. et al. Recognition of methylated DNA by TAL effectors. Cell Res. 22, 1502–1504 (2012).
Gao, H., Wu, X., Chai, J. & Han, Z. Crystal structure of a TALE protein reveals an extended N-terminal DNA binding region. Cell Res. 22, 1716–1720 (2012).
Kemen, E. et al. Identification of a protein from rust fungi transferred from haustoria into infected plant cells. Mol. Plant Microbe Interact. 18, 1130–1139 (2005).
Khang, C. H. et al. Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell 22, 1388–1403 (2010).
Panstruga, R. & Dodds, P. N. Terrific protein traffic: the mystery of effector protein delivery by filamentous plant pathogens. Science 324, 748–750 (2009).
Ellis, J. G. & Dodds, P. N. Showdown at the RXLR motif: Serious differences of opinion in how effector proteins from filamentous eukaryotic pathogens enter plant cells. Proc. Natl Acad. Sci. USA 108, 14381–14382 (2011). This paper provides a rational summary of the contrasting opinions in the debate on translocation of oomycete and fungal effectors.
Tyler, B. M. et al. Microbe-independent entry of oomycete RxLR effectors and fungal RxLR-like effectors into plant and animal cells is specific and reproducible. Mol. Plant Microbe Interact. 26, 611–616 (2013).
Wawra, S. et al. In vitro translocation experiments with RxLR-reporter fusion proteins of Avr1b from Phytophthora sojae and AVR3a from Phytophthora infestans fail to demonstrate specific autonomous uptake in plant and animal cells. Mol. Plant Microbe Interact. 26, 528–536 (2013).
Boutemy, L. S. et al. Structures of Phytophthora RXLR effector proteins: a conserved but adaptable fold underpins functional diversity. J. Biol. Chem. 286, 35834–35842 (2011). This paper shows crystal structures of two RXLR effectors (Avr3a11 and PexRD2), which adopt a similar helical WY fold despite sharing less than 20% sequence similarity.
Yaeno, T. et al. Phosphatidylinositol monophosphate-binding interface in the oomycete RXLR effector AVR3a is required for its stability in host cells to modulate plant immunity. Proc. Natl Acad. Sci. USA 108, 14682–14687 (2011). This article shows the NMR structure of an RXLR effector of the Avr3a family, which provides evidence for phospholipid binding of the C-terminal effector domain.
Sun, F. et al. Structural basis for interactions of the Phytophthora sojae RxLR effector Avh5 with phosphatidylinositol 3-phosphate and for host cell entry. Mol. Plant Microbe Interact. 26, 330–344 (2013).
Bos, J. I. B. et al. The C-terminal half of Phytophthora infestans RXLR effector AVR3a is sufficient to trigger R3a-mediated hypersensitivity and suppress INF1-induced cell death in Nicotiana benthamiana. Plant J. 48, 165–176 (2006).
Win, J. et al. Adaptive evolution has targeted the C-terminal domain of the RXLR effectors of plant pathogenic oomycetes. Plant Cell 19, 2349–2369 (2007).
Chou, S. et al. Hyaloperonospora arabidopsidis ATR1 effector is a repeat protein with distributed recognition surfaces. Proc. Natl Acad. Sci. USA 108, 13323–13328 (2011). In this paper, the crystal structure of ATR1 provides insight into how WY domains are arranged in effectors containing more than one WY repeat.
Leonelli, L. et al. Structural elucidation and functional characterization of the Hyaloperonospora arabidopsidis effector protein ATR13. PLoS Pathog. 7, e1002428 (2011).
Win, J. et al. Sequence divergent RXLR effectors share a structural fold conserved across plant pathogenic oomycete species. PLoS Pathog. 8, e1002400 (2012).
Stergiopoulos, I. & de Wit, P. J. Fungal effector proteins. Annu. Rev. Phytopathol. 47, 233–263 (2009).
Wang, C. I. et al. Crystal structures of flax rust avirulence proteins AvrL567-A and -D reveal details of the structural basis for flax disease resistance specificity. Plant Cell 19, 2898–2912 (2007). The crystal structures of two members of the AvrL567 group of flax rust effector proteins that are recognized by direct binding to their corresponding NB-LRR proteins are shown in this paper.
Zhang, Z. M. et al. Solution structure of the Magnaporthe oryzae avirulence protein AvrPiz-t. J. Biomol. NMR 55, 219–223 (2013).
Dodds, P. N. et al. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc. Natl Acad. Sci. USA 103, 8888–8893 (2006).
Catanzariti, A. M. et al. The AvrM effector from flax rust has a structured C-terminal domain and interacts directly with the M resistance protein. Mol. Plant Microbe Interact. 23, 49–57 (2010).
Krasileva, K. V., Dahlbeck, D. & Staskawicz, B. J. Activation of an Arabidopsis resistance protein is specified by the in planta association of its leucine-rich repeat domain with the cognate oomycete effector. Plant Cell 22, 2444–2458 (2010).
Heidrich, K., Blanvillain-Baufume, S. & Parker, J. E. Molecular and spatial constraints on NB-LRR receptor signaling. Curr. Opin. Plant Biol. 15, 385–391 (2012).
Collier, S. M. & Moffett, P. NB-LRRs work a “bait and switch” on pathogens. Trends Plant Sci. 14, 521–529 (2009).
Maekawa, T., Kufer, T. A. & Schulze-Lefert, P. NLR functions in plant and animal immune systems: so far and yet so close. Nature Immunol. 12, 817–826 (2011).
Maekawa, T. et al. Coiled-coil domain-dependent homodimerization of intracellular barley immune receptors defines a minimal functional module for triggering cell death. Cell Host Microbe 9, 187–199 (2011). In this paper, the crystal structure of the N-terminal CC domain of the barley MLA10 immune receptor shows a domain-swapped homodimer, which suggests that dimerization of the CC domain creates the molecular interface for binding of a WRKY-type transcription factor.
Shen, Q. H. et al. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 315, 1098–1103 (2007).
Chan, S. L., Mukasa, T., Santelli, E., Low, L. Y. & Pascual, J. The crystal structure of a TIR domain from Arabidopsis thaliana reveals a conserved helical region unique to plants. Protein Sci. 19, 155–161 (2010).
Bernoux, M. et al. Structural and functional analysis of a plant resistance protein TIR domain reveals interfaces for self-association, signaling, and autoregulation. Cell Host Microbe 9, 200–211 (2011). In this paper, the crystal structure of the TIR domain from the flax L6 immune receptor shows an interface for homodimerization of TIR domains and that residues contributing to this interface are essential for L6 function.
Krasileva, K. V., Dahlbeck, D. & Staskawicz, B. J. Activation of an Arabidopsis resistance protein Is specified by the in planta association of its leucine-rich repeat domain with the cognate oomycete effector. Plant Cell 22, 2444–2458 (2010).
Mestre, P. & Baulcombe, D. C. Elicitor-mediated oligomerization of the tobacco N disease resistance protein. Plant Cell 18, 491–501 (2006).
Ausubel, F. M. Are innate immune signaling pathways in plants and animals conserved? Nature Immunol. 6, 973–979 (2005).
Spoel, S. H. & Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nature Rev. Immunol. 12, 89–100 (2012).
Faulkner, C. & Robatzek, S. Plants and pathogens: putting infection strategies and defence mechanisms on the map. Curr. Opin. Plant Biol. 15, 699–707 (2012).
Monaghan, J. & Zipfel, C. Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 15, 349–357 (2012).
Whisson, S. C. et al. A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature 450, 115–118 (2007). This work establishes that the RXLR motif is essential for host cell delivery of the oomycete effector Avr3a.
Coll, N. S., Epple, P. & Dangl, J. L. Programmed cell death in the plant immune system. Cell Death Differ. 18, 1247–1256 (2011).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Plechanovova, A., Jaffray, E. G., Tatham, M. H., Naismith, J. H. & Hay, R. T. Structure of a RING E3 ligase and ubiquitin-loaded E2 primed for catalysis. Nature 489, 115–120 (2012).
Garcia-Pino, A. et al. Doc of prophage P1 is inhibited by its antitoxin partner Phd through fold complementation. J. Biol. Chem. 283, 30821–30827 (2008).
Hu, Z. et al. Crystal structure of NLRC4 reveals its autoinhibition mechanism. Science 341, 172–175 (2013).
Sanchez-Vallet, A. et al. Fungal effector Ecp6 outcompetes host immune receptor for chitin binding through intrachain LysM dimerization. eLife 2, e00790 (2013).
Acknowledgements
The authors thank S. Schornack and C. Zipfel for comments during the preparation of this manuscript. L.W. acknowledges support from Federation of the European Biochemical Societies (long-term fellowship) and the Biotechnology and Biological Sciences Research Council (BBSRC; grant BB/K009176/1). Work in the M.J.B. laboratory, relevant to the areas discussed, is funded by the BBSRC (grants BB/J004553/1 and BB/I019557/1), the European Research Council (proposal 294608), the John Innes Foundation and the Gatsby Charitable Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Apoplastic effectors
-
Effectors that are secreted into and act in the apoplast, a tissue-level compartment outside the plant plasma membrane that includes the cell wall.
- Cytoplasmic effectors
-
Effectors that are secreted and translocated across the plant plasma membrane into the host cytoplasm, where they can target different subcellular compartments.
- Necrotrophic
-
An organism that kills host cells before invasion and gains nutrients from the dead plant tissue.
- Hemibiotrophic
-
An organism that feeds on living tissues for a period and then switches to necrotrophic colonization of dead tissues.
- Obligate biotrophic
-
An organism that can only complete its life cycle on living plant tissue; such organisms actively prevent host cell death and feed on living plant tissue.
- Hypersensitive response
-
(HR). A specific form of programmed cell death, often induced by effector-triggered immunity and correlated with accumulation of antimicrobial compounds and systemic acquired resistance.
- Pathovars
-
Pathogenic variants within a species that are defined by a characteristic host range and/or tissue specificity.
- E3 ligases
-
Enzymes required to attach the molecular tag ubiquitin to proteins. This tag modifies protein function or targets the protein for proteosomal degradation.
- Salicylic acid
-
Also known as salicylate, this is a central plant hormone signal that induces local and systemic defence responses in plants, collectively known as systemic acquired resistance.
- Ethylene
-
A gaseous, unsaturated hydrocarbon that acts as a plant hormone to promote growth and development and as an inhibiting stress factor.
Rights and permissions
About this article
Cite this article
Wirthmueller, L., Maqbool, A. & Banfield, M. On the front line: structural insights into plant–pathogen interactions. Nat Rev Microbiol 11, 761–776 (2013). https://doi.org/10.1038/nrmicro3118
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3118
This article is cited by
-
A cell-free approach to identify binding hotspots in plant immune receptors
Scientific Reports (2022)
-
Salicylic acid is a key player of Arabidopsis autophagy mutant susceptibility to the necrotrophic bacterium Dickeya dadantii
Scientific Reports (2021)
-
Identification of the key genes involved in the regulation of symbiotic pathways induced by Metarhizium anisopliae in peanut (Arachis hypogaea) roots
3 Biotech (2020)
-
Characterization of phytohormone and transcriptome reprogramming profiles during maize early kernel development
BMC Plant Biology (2019)
-
Structural genomics applied to the rust fungus Melampsora larici-populina reveals two candidate effector proteins adopting cystine knot and NTF2-like protein folds
Scientific Reports (2019)