Key Points
-
The post-translational general secretion (Sec) pathway is powered by the essential ATPase SecA, which works with the SecYEG channel to translocate proteins across the cytoplasmic membrane.
-
Mycobacteria and some Gram-positive bacteria have two non-redundant SecA proteins, SecA1 and SecA2. SecA1 powers the essential canonical Sec pathway, whereas SecA2 mediates export of a limited set of proteins.
-
SecA2 export systems have diverse and important roles in cell envelope biogenesis and bacterial pathogenesis.
-
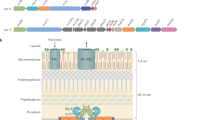
There are two types of SecA2 systems: SecA2–SecY2 systems and SecA2-only systems.
-
SecA2–SecY2 systems seem to operate mostly independent of the canonical Sec machinery as dedicated transporters of a group of serine-rich glycoproteins that function as adhesins. SecA2–SecY2 systems are encoded by a genomic locus that includes ORFs for glycosylation and export machinery.
-
SecA2-only systems do not contain a SecY2 or an obvious accessory membrane channel. Instead, SecA2-only systems seem to use the canonical SecYEG channel for exporting a diverse assortment of proteins.
-
It is unknown how SecA2 functions in comparison to the well-studied canonical SecA1 proteins. The same structural, biochemical and in vitro reconstitution analyses that have been used to understand canonical Sec-mediated export will surely prove valuable in answering the many mechanistic questions concerning SecA2–SecY2 and SecA2-only systems.
Abstract
The conserved general secretion (Sec) pathway carries out most protein export in bacteria and is powered by the essential ATPase SecA. Interestingly, mycobacteria and some Gram-positive bacteria possess two SecA proteins: SecA1 and SecA2. In these species, SecA1 is responsible for exporting most proteins, whereas SecA2 exports only a subset of substrates and is implicated in virulence. However, despite the impressive body of knowledge about the canonical SecA1, less is known concerning SecA2 function. Here, we review our current understanding of the different types of SecA2 systems and outline future directions for their study.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
du Plessis, D. J., Nouwen, N. & Driessen, A. J. The Sec translocase. Biochim. Biophys. Acta 1808, 851–865 (2011).
Van den Berg, B. et al. X-ray structure of a protein-conducting channel. Nature 427, 36–44 (2004).
Taura, T., Baba, T., Akiyama, Y. & Ito, K. Determinants of the quantity of the stable SecY complex in the Escherichia coli cell. J. Bacteriol. 175, 7771–7775 (1993).
Harris, C. R. & Silhavy, T. J. Mapping an interface of SecY (PrlA) and SecE (PrlG) by using synthetic phenotypes and in vivo cross-linking. J. Bacteriol. 181, 3438–3444 (1999).
Tam, P. C., Maillard, A. P., Chan, K. K. & Duong, F. Investigating the SecY plug movement at the SecYEG translocation channel. EMBO J. 24, 3380–3388 (2005).
Duong, F. & Wickner, W. The PrlA and PrlG phenotypes are caused by a loosened association among the translocase SecYEG subunits. EMBO J. 18, 3263–3270 (1999).
Nishiyama, K., Mizushima, S. & Tokuda, H. Preferential interaction of Sec-G with Sec-E stabilizes an unstable Sec-E derivative in the Escherichia coli cytoplasmic membrane. Biochem. Biophys. Res. Commun. 217, 217–223 (1995).
Nishiyama, K., Suzuki, T. & Tokuda, H. Inversion of the membrane topology of SecG coupled with SecA-dependent preprotein translocation. Cell 85, 71–81 (1996).
Xie, K. & Dalbey, R. E. Inserting proteins into the bacterial cytoplasmic membrane using the Sec and YidC translocases. Nature Rev. Microbiol. 6, 234–244 (2008).
Shimohata, N., Akiyama, Y. & Ito, K. Peculiar properties of DsbA in its export across the Escherichia coli cytoplasmic membrane. J. Bacteriol. 187, 3997–4004 (2005).
Valent, Q. A. et al. Nascent membrane and presecretory proteins synthesized in Escherichia coli associate with signal recognition particle and trigger factor. Mol. Microbiol. 25, 53–64 (1997).
Egea, P. F. & Stroud, R. M. Lateral opening of a translocon upon entry of protein suggests the mechanism of insertion into membranes. Proc. Natl Acad. Sci. USA 107, 17182–17187 (2010).
von Heijne, G. The signal peptide. J. Membr. Biol. 115, 195–201 (1990).
Bechtluft, P., Nouwen, N., Tans, S. J. & Driessen, A. J. SecB—a chaperone dedicated to protein translocation. Mol. Biosyst. 6, 620–627 (2010).
Fisher, A. C. & DeLisa, M. P. A little help from my friends: quality control of presecretory proteins in bacteria. J. Bacteriol. 186, 7467–7473 (2004).
Arkowitz, R. A., Joly, J. C. & Wickner, W. Translocation can drive the unfolding of a preprotein domain. EMBO J. 12, 243–253 (1993).
Nouwen, N., Berrelkamp, G. & Driessen, A. J. Bacterial Sec-translocase unfolds and translocates a class of folded protein domains. J. Mol. Biol. 372, 422–433 (2007).
Kusters, I. & Driessen, A. J. SecA, a remarkable nanomachine. Cell. Mol. Life Sci. 68, 2053–2066 (2011). A comprehensive review on the structure and function of SecA proteins.
Brundage, L., Hendrick, J. P., Schiebel, E., Driessen, A. J. & Wickner, W. The purified E. coli integral membrane protein SecY/E is sufficient for reconstitution of SecA-dependent precursor protein translocation. Cell 62, 649–657 (1990).
Economou, A. & Wickner, W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell 78, 835–843 (1994).
Oliver, D. B. & Beckwith, J. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell 25, 765–772 (1981). References 19–21 are among a collection of seminal papers describing SecA-dependent protein export.
Papanikou, E. et al. Identification of the preprotein binding domain of SecA. J. Biol. Chem. 280, 43209–43217 (2005).
Gelis, I. et al. Structural basis for signal-sequence recognition by the translocase motor SecA as determined by NMR. Cell 131, 756–769 (2007).
Musial-Siwek, M., Rusch, S. L. & Kendall, D. A. Selective photoaffinity labeling identifies the signal peptide binding domain on SecA. J. Mol. Biol. 365, 637–648 (2007).
Auclair, S. M. et al. Mapping of the signal peptide-binding domain of Escherichia coli SecA using Forster resonance energy transfer. Biochemistry 49, 782–792 (2010).
Kimura, E., Akita, M., Matsuyama, S. & Mizushima, S. Determination of a region in SecA that interacts with presecretory proteins in Escherichia coli. J. Biol. Chem. 266, 6600–6606 (1991).
Mori, H. & Ito, K. Different modes of SecY–SecA interactions revealed by site-directed in vivo photo-cross-linking. Proc. Natl Acad. Sci. USA 103, 16159–16164 (2006).
van der Sluis, E. O. et al. Identification of two interaction sites in SecY that are important for the functional interaction with SecA. J. Mol. Biol. 361, 839–849 (2006).
Breukink, E. et al. The C terminus of SecA is involved in both lipid binding and SecB binding. J. Biol. Chem. 270, 7902–7907 (1995).
Li, W. et al. The plug domain of the SecY protein stabilizes the closed state of the translocation channel and maintains a membrane seal. Mol. Cell 26, 511–521 (2007).
Smith, M. A., Clemons, W. M. Jr, DeMars, C. J. & Flower, A. M. Modeling the effects of prl mutations on the Escherichia coli SecY complex. J. Bacteriol. 187, 6454–6465 (2005).
Gouridis, G., Karamanou, S., Gelis, I., Kalodimos, C. G. & Economou, A. Signal peptides are allosteric activators of the protein translocase. Nature 462, 363–367 (2009).
van der Wolk, J. P., de Wit, J. G. & Driessen, A. J. The catalytic cycle of the Escherichia coli SecA ATPase comprises two distinct preprotein translocation events. EMBO J. 16, 7297–7304 (1997).
Paetzel, M., Karla, A., Strynadka, N. C. & Dalbey, R. E. Signal peptidases. Chem. Rev. 102, 4549–4580 (2002).
Oliver, D., Kumamoto, C., Quinlan, M. & Beckwith, J. Pleiotropic mutants affecting the secretory apparatus of Escherichia coli. Ann. Microbiol. (Paris) 133, 105–110 (1982).
Murphy, C. K., Stewart, E. J. & Beckwith, J. A double counter-selection system for the study of null alleles of essential genes in Escherichia coli. Gene 155, 1–7 (1995).
Nishiyama, K., Hanada, M. & Tokuda, H. Disruption of the gene encoding p12 (SecG) reveals the direct involvement and important function of SecG in the protein translocation of Escherichia coli at low temperature. EMBO J. 13, 3272–3277 (1994).
Pogliano, J. A. & Beckwith, J. SecD and SecF facilitate protein export in Escherichia coli. EMBO J. 13, 554–561 (1994).
Duong, F. & Wickner, W. Distinct catalytic roles of the SecYE, SecG and SecDFYajC subunits of preprotein translocase holoenzyme. EMBO J. 16, 2756–2768 (1997).
Economou, A. Following the leader: bacterial protein export through the Sec pathway. Trends Microbiol. 7, 315–320 (1999).
Cole, S. T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998).
Braunstein, M., Brown, A. M., Kurtz, S. & Jacobs, W. R. Jr . Two nonredundant SecA homologues function in mycobacteria. J. Bacteriol. 183, 6979–6990 (2001).
Rigel, N. W. & Braunstein, M. A new twist on an old pathway – accessory Sec systems. Mol. Microbiol. 69, 291–302 (2008). A review of SecA2 export, including a phylogenetic tree that depicts the evolutionary relationship of SecA2 proteins from both SecA2-only and SecA2–SecY2 systems.
Bensing, B. A. & Sullam, P. M. Characterization of Streptococcus gordonii SecA2 as a paralogue of SecA. J. Bacteriol. 191, 3482–3491 (2009).
Rigel, N. W. et al. The accessory SecA2 system of mycobacteria requires ATP binding and the canonical SecA1. J. Biol. Chem. 284, 9927–9936 (2009). The first demonstration that a SecA2-only system requires one or more components of the canonical Sec pathway.
Guo, X. V. et al. Silencing Mycobacterium smegmatis by using tetracycline repressors. J. Bacteriol. 189, 4614–4623 (2007).
Fagan, R. P. & Fairweather, N. F. Clostridium difficile has two parallel and essential Sec secretion systems. J. Biol. Chem. 286, 27483–27493 (2011). Confirmation that the SecA2-only system from C. difficile requires one or more components of the canonical Sec pathway.
Caspers, M. & Freudl, R. Corynebacterium glutamicum possesses two secA homologous genes that are essential for viability. Arch. Microbiol. 189, 605–610 (2008).
Braunstein, M., Espinosa, B. J., Chan, J., Belisle, J. T. & Jacobs, W. R. Jr. SecA2 functions in the secretion of superoxide dismutase A and in the virulence of Mycobacterium tuberculosis. Mol. Microbiol. 48, 453–464 (2003).
Kurtz, S., McKinnon, K. P., Runge, M. S., Ting, J. P. & Braunstein, M. The SecA2 secretion factor of Mycobacterium tuberculosis promotes growth in macrophages and inhibits the host immune response. Infect. Immun. 74, 6855–6864 (2006).
Sullivan, J. T., Young, E. F., McCann, J. R. & Braunstein, M. The Mycobacterium tuberculosis SecA2 system subverts phagosome maturation to promote growth in macrophages. Infect. Immun. 80, 996–1006 (2012).
Xiong, Y. Q., Bensing, B. A., Bayer, A. S., Chambers, H. F. & Sullam, P. M. Role of the serine-rich surface glycoprotein GspB of Streptococcus gordonii in the pathogenesis of infective endocarditis. Microb. Pathog. 45, 297–301 (2008).
Wu, H., Mintz, K. P., Ladha, M. & Fives-Taylor, P. M. Isolation and characterization of Fap1, a fimbriae-associated adhesin of Streptococcus parasanguis FW213. Mol. Microbiol. 28, 487–500 (1998). References 52 and 53 show that SecA2–SecY2 systems export proteins which are first glycosylated in the cytoplasm.
Siboo, I. R., Chambers, H. F. & Sullam, P. M. Role of SraP, a serine-rich surface protein of Staphylococcus aureus, in binding to human platelets. Infect. Immun. 73, 2273–2280 (2005).
Lenz, L. L., Mohammadi, S., Geissler, A. & Portnoy, D. A. SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis. Proc. Natl Acad. Sci. USA 100, 12432–12437 (2003).
Sardis, M. F. & Economou, A. SecA: a tale of two protomers. Mol. Microbiol. 76, 1070–1081 (2010).
Sharma, V. et al. Crystal structure of Mycobacterium tuberculosis SecA, a preprotein translocating ATPase. Proc. Natl Acad. Sci. USA 100, 2243–2248 (2003).
Hunt, J. F. et al. Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science 297, 2018–2026 (2002).
Papanikolau, Y. et al. Structure of dimeric SecA, the Escherichia coli preprotein translocase motor. J. Mol. Biol. 366, 1545–1557 (2007).
Sianidis, G. et al. Cross-talk between catalytic and regulatory elements in a DEAD motor domain is essential for SecA function. EMBO J. 20, 961–970 (2001).
Keramisanou, D. et al. Disorder-order folding transitions underlie catalysis in the helicase motor of SecA. Nature Struct. Mol. Biol. 13, 594–602 (2006).
Hou, J. M. et al. ATPase activity of Mycobacterium tuberculosis SecA1 and SecA2 proteins and its importance for SecA2 function in macrophages. J. Bacteriol. 190, 4880–4887 (2008).
Fekkes, P. et al. Preprotein transfer to the Escherichia coli translocase requires the co-operative binding of SecB and the signal sequence to SecA. Mol. Microbiol. 29, 1179–1190 (1998).
Fekkes, P., van der Does, C. & Driessen, A. J. The molecular chaperone SecB is released from the carboxy-terminus of SecA during initiation of precursor protein translocation. EMBO J. 16, 6105–6113 (1997).
Zhou, J. & Xu, Z. Structural determinants of SecB recognition by SecA in bacterial protein translocation. Nature Struct. Biol. 10, 942–947 (2003).
Fekkes, P., de Wit, J. G., Boorsma, A., Friesen, R. H. & Driessen, A. J. Zinc stabilizes the SecB binding site of SecA. Biochemistry 38, 5111–5116 (1999).
van Wely, K. H., Swaving, J., Freudl, R. & Driessen, A. J. Translocation of proteins across the cell envelope of Gram-positive bacteria. FEMS Microbiol. Rev. 25, 437–454 (2001).
Bensing, B. A. & Sullam, P. M. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44, 1081–1094 (2002).
Mistou, M. Y., Dramsi, S., Brega, S., Poyart, C. & Trieu-Cuot, P. Molecular dissection of the secA2 locus of group B Streptococcus reveals that glycosylation of the Srr1 LPXTG protein is required for full virulence. J. Bacteriol. 191, 4195–4206 (2009).
Chen, Q., Wu, H. & Fives-Taylor, P. M. Investigating the role of secA2 in secretion and glycosylation of a fimbrial adhesin in Streptococcus parasanguis FW213. Mol. Microbiol. 53, 843–856 (2004).
Obert, C. et al. Identification of a candidate Streptococcus pneumoniae core genome and regions of diversity correlated with invasive pneumococcal disease. Infect. Immun. 74, 4766–4777 (2006).
Siboo, I. R., Chaffin, D. O., Rubens, C. E. & Sullam, P. M. Characterization of the accessory Sec system of Staphylococcus aureus. J. Bacteriol. 190, 6188–6196 (2008).
Samen, U., Eikmanns, B. J., Reinscheid, D. J. & Borges, F. The surface protein Srr-1 of Streptococcus agalactiae binds human keratin 4 and promotes adherence to epithelial HEp-2 cells. Infect. Immun. 75, 5405–5414 (2007).
Wu, H. & Fives-Taylor, P. M. Identification of dipeptide repeats and a cell wall sorting signal in the fimbriae-associated adhesin, Fap1, of Streptococcus parasanguis. Mol. Microbiol. 34, 1070–1081 (1999).
Bensing, B. A., Gibson, B. W. & Sullam, P. M. The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export. J. Bacteriol. 186, 638–645 (2004).
Takamatsu, D., Bensing, B. A. & Sullam, P. M. Four proteins encoded in the gspB-secY2A2 operon of Streptococcus gordonii mediate the intracellular glycosylation of the platelet-binding protein GspB. J. Bacteriol. 186, 7100–7111 (2004).
Bu, S. et al. Interaction between two putative glycosyltransferases is required for glycosylation of a serine-rich streptococcal adhesin. J. Bacteriol. 190, 1256–1266 (2008).
Wu, H., Bu, S., Newell, P., Chen, Q. & Fives-Taylor, P. Two gene determinants are differentially involved in the biogenesis of Fap1 precursors in Streptococcus parasanguis. J. Bacteriol. 189, 1390–1398 (2007).
Takamatsu, D., Bensing, B. A. & Sullam, P. M. Genes in the accessory sec locus of Streptococcus gordonii have three functionally distinct effects on the expression of the platelet-binding protein GspB. Mol. Microbiol. 52, 189–203 (2004).
Zhou, M., Zhu, F., Dong, S., Pritchard, D. G. & Wu, H. A novel glucosyltransferase is required for glycosylation of a serine-rich adhesin and biofilm formation by Streptococcus parasanguinis. J. Biol. Chem. 285, 12140–12148 (2010).
Wu, H., Zeng, M. & Fives-Taylor, P. The glycan moieties and the N-terminal polypeptide backbone of a fimbria-associated adhesin, Fap1, play distinct roles in the biofilm development of Streptococcus parasanguinis. Infect. Immun. 75, 2181–2188 (2007).
Bensing, B. A., Lopez, J. A. & Sullam, P. M. The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibα. Infect. Immun. 72, 6528–6537 (2004).
Stephenson, A. E. et al. The Fap1 fimbrial adhesin is a glycoprotein: antibodies specific for the glycan moiety block the adhesion of Streptococcus parasanguis in an in vitro tooth model. Mol. Microbiol. 43, 147–157 (2002).
Seepersaud, R., Bensing, B. A., Yen, Y. T. & Sullam, P. M. Asp3 mediates multiple protein-protein interactions within the accessory Sec system of Streptococcus gordonii. Mol. Microbiol. 78, 490–505 (2010). The finding that glycosylation of a SecA2–SecY2 substrate blocks export by the canonical SecA1–SecYEG pathway.
Takamatsu, D., Bensing, B. A. & Sullam, P. M. Two additional components of the accessory Sec system mediating export of the Streptococcus gordonii platelet-binding protein GspB. J. Bacteriol. 187, 3878–3883 (2005).
Peng, Z. et al. Role of gap3 in Fap1 glycosylation, stability, in vitro adhesion, and fimbrial and biofilm formation of Streptococcus parasanguinis. Oral Microbiol. Immunol. 23, 70–78 (2008).
Li, Y. et al. A conserved domain of previously unknown function in Gap1 mediates protein–protein interaction and is required for biogenesis of a serine-rich streptococcal adhesin. Mol. Microbiol. 70, 1094–1104 (2008).
Zhou, M., Zhang, H., Zhu, F. & Wu, H. Canonical SecA associates with an accessory secretory protein complex involved in biogenesis of a streptococcal serine-rich repeat glycoprotein. J. Bacteriol. 193, 6560–6566 (2011).
Zhou, M., Zhu, F., Li, Y., Zhang, H. & Wu, H. Gap1 functions as a molecular chaperone to stabilize its interactive partner Gap3 during biogenesis of serine-rich repeat bacterial adhesin. Mol. Microbiol. 83, 866–878 (2012).
Yen, Y. T., Seepersaud, R., Bensing, B. A. & Sullam, P. M. Asp2 and Asp3 interact directly with GspB, the export substrate of the Streptococcus gordonii accessory Sec System. J. Bacteriol. 193, 3165–3174 (2011).
Bensing, B. A., Takamatsu, D. & Sullam, P. M. Determinants of the streptococcal surface glycoprotein GspB that facilitate export by the accessory Sec system. Mol. Microbiol. 58, 1468–1481 (2005).
Chen, Q., Sun, B., Wu, H., Peng, Z. & Fives-Taylor, P. M. Differential roles of individual domains in selection of secretion route of a Streptococcus parasanguinis serine-rich adhesin, Fap1. J. Bacteriol. 189, 7610–7617 (2007).
Nita-Lazar, M., Wacker, M., Schegg, B., Amber, S. & Aebi, M. The N-X-S/T consensus sequence is required but not sufficient for bacterial N-linked protein glycosylation. Glycobiology 15, 361–367 (2005).
VanderVen, B. C., Harder, J. D., Crick, D. C. & Belisle, J. T. Export-mediated assembly of mycobacterial glycoproteins parallels eukaryotic pathways. Science 309, 941–943 (2005).
Schwarz, F. & Aebi, M. Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 21, 576–582 (2011).
Grass, S. et al. The Haemophilus influenzae HMW1 adhesin is glycosylated in a process that requires HMW1C and phosphoglucomutase, an enzyme involved in lipooligosaccharide biosynthesis. Mol. Microbiol. 48, 737–751 (2003).
Choi, K. J. et al. The Actinobacillus pleuropneumoniae HMW1C-like glycosyltransferase mediates N-linked glycosylation of the Haemophilus influenzae HMW1 adhesin. PLoS ONE 5, e15888 (2010).
Charbonneau, M. E. & Mourez, M. The Escherichia coli AIDA-I autotransporter undergoes cytoplasmic glycosylation independently of export. Res. Microbiol. 159, 537–544 (2008).
Bonardi, F. et al. Probing the SecYEG translocation pore size with preproteins conjugated with sizable rigid spherical molecules. Proc. Natl Acad. Sci. USA 108, 7775–7780 (2011).
Bensing, B. A., Siboo, I. R. & Sullam, P. M. Glycine residues in the hydrophobic core of the GspB signal sequence route export toward the accessory Sec pathway. J. Bacteriol. 189, 3846–3854 (2007).
Bensing, B. A. & Sullam, P. M. Transport of preproteins by the accessory Sec system requires a specific domain adjacent to the signal peptide. J. Bacteriol. 192, 4223–4232 (2010).
Sibbald, M. J. et al. Synthetic effects of secG and secY2 mutations on exoproteome biogenesis in Staphylococcus aureus. J. Bacteriol. 192, 3788–3800 (2010).
Lenz, L. L. & Portnoy, D. A. Identification of a second Listeria secA gene associated with protein secretion and the rough phenotype. Mol. Microbiol. 45, 1043–1056 (2002). The first description of SecA2 and an associated exported substrate in Listeria spp.
Sadagopal, S. et al. Reducing the activity and secretion of microbial antioxidants enhances the immunogenicity of BCG. PLoS ONE 4, e5531 (2009).
Halbedel, S., Hahn, B., Daniel, R. A. & Flieger, A. DivIVA affects secretion of virulence-related autolysins in Listeria monocytogenes. Mol. Microbiol. 83, 821–839 (2012).
Mishra, K. K., Mendonca, M., Aroonnual, A., Burkholder, K. M. & Bhunia, A. K. Genetic organization and molecular characterization of secA2 locus in Listeria species. Gene 489, 76–85 (2011).
Machata, S., Hain, T., Rohde, M. & Chakraborty, T. Simultaneous deficiency of both MurA and p60 proteins generates a rough phenotype in Listeria monocytogenes. J. Bacteriol. 187, 8385–8394 (2005).
Burkholder, K. M. et al. Expression of LAP, a SecA2-dependent secretory protein, is induced under anaerobic environment. Microbes Infect. 11, 859–867 (2009).
Hinchey, J. et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Invest. 117, 2279–2288 (2007).
Hinchey, J. et al. Lysine auxotrophy combined with deletion of the secA2 gene results in a safe and highly immunogenic candidate live attenuated vaccine for tuberculosis. PLoS ONE 6, e15857 (2011).
Rahmoun, M. et al. Priming of protective anti-Listeria monocytogenes memory CD8+ T cells requires a functional SecA2 secretion system. Infect. Immun. 79, 2396–2403 (2011).
Gibbons, H. S., Wolschendorf, F., Abshire, M., Niederweis, M. & Braunstein, M. Identification of two Mycobacterium smegmatis lipoproteins exported by a SecA2-dependent pathway. J. Bacteriol. 189, 5090–5100 (2007).
Archambaud, C., Nahori, M. A., Pizarro-Cerda, J., Cossart, P. & Dussurget, O. Control of Listeria superoxide dismutase by phosphorylation. J. Biol. Chem. 281, 31812–31822 (2006).
Calabi, E. et al. Molecular characterization of the surface layer proteins from Clostridium difficile. Mol. Microbiol. 40, 1187–1199 (2001).
Fagan, R. P. et al. A proposed nomenclature for cell wall proteins of Clostridium difficile. J. Med. Microbiol. 60, 1225–1228 (2011).
Waligora, A. J. et al. Characterization of a cell surface protein of Clostridium difficile with adhesive properties. Infect. Immun. 69, 2144–2153 (2001).
Calabi, E., Calabi, F., Phillips, A. D. & Fairweather, N. F. Binding of Clostridium difficile surface layer proteins to gastrointestinal tissues. Infect. Immun. 70, 5770–5778 (2002).
Janoir, C., Pechine, S., Grosdidier, C. & Collignon, A. Cwp84, a surface-associated protein of Clostridium difficile, is a cysteine protease with degrading activity on extracellular matrix proteins. J. Bacteriol. 189, 7174–7180 (2007).
Bramkamp, M. & van Baarle, S. Division site selection in rod-shaped bacteria. Curr. Opin. Microbiol. 12, 683–688 (2009).
Zimmer, J., Nam, Y. & Rapoport, T. A. Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature 455, 936–943 (2008). A landmark structural paper showing SecA bound to SecY.
Erlandson, K. J. et al. A role for the two-helix finger of the SecA ATPase in protein translocation. Nature 455, 984–987 (2008).
Mitchell, C. & Oliver, D. Two distinct ATP-binding domains are needed to promote protein export by Escherichia coli SecA ATPase. Mol. Microbiol. 10, 483–497 (1993).
Ye, J., Osborne, A. R., Groll, M. & Rapoport, T. A. RecA-like motor ATPases—lessons from structures. Biochim. Biophys. Acta 1659, 1–18 (2004).
Karamanou, S. et al. A molecular switch in SecA protein couples ATP hydrolysis to protein translocation. Mol. Microbiol. 34, 1133–1145 (1999).
Vrontou, E., Karamanou, S., Baud, C., Sianidis, G. & Economou, A. Global co-ordination of protein translocation by the SecA IRA1 switch. J. Biol. Chem. 279, 22490–22497 (2004).
Acknowledgements
The authors thank members of the Braunstein laboratory for critical reading of this manuscript and N. Rigel for assistance with the graphic of the SecA1 structure in figure 2. Work on SecA2 in the Braunstein laboratory is supported by the US National Institutes of Health grant AI054540.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Preproteins
-
Proteins that are synthesized with amino-terminal signal peptides for targeting to a particular cellular location.
- Signal peptide
-
An amino-terminal amino acid sequence that is present on preproteins. The signal peptide helps target specific proteins for export out of the bacterial cytoplasm. Sec signal peptides are composed of a tripartite structure with a positively charged N terminus, a hydrophobic core and a signal-peptidase-cleavage site.
- In vitro reconstitution
-
A technique for studying biochemical processes in vitro. Reconstitution of the Sec pathway in vitro involves incubation of preprotein and purified SecA with SecYEG-containing inverted membrane vesicles (IMVs). Preprotein translocation through SecYEG into an IMV is monitored by the loss of preprotein sensitivity to protease.
- Glycosyltransferases
-
Enzymes that catalyse the post-translational addition of carbohydrate mono- or oligosaccharides onto acceptor molecules. Some glycosyl-transferases attach sugars to proteins to form glycoproteins. The sugar composition and structure of glycosyl modifications can be diverse.
- O-linked glycosylation
-
A type of glycosylation in which a saccharide moiety is added to the hydroxyl oxygen of a serine or threonine residue.
- Phagosome maturation
-
The process by which phagosomal vacuoles form around foreign particles or bacteria within a eukaryotic cell and ultimately fuse with lysosomes, resulting in a degradative phagolysosomal compartment.
- Autolysin
-
A hydrolase that breaks the peptidoglycan matrix in the bacterial cell wall. Autolysins are important in bacterial cell growth and division.
- S-layer
-
(Surface layer). An ordered array of protein subunits that form a lattice structure surrounding the bacterial cell wall. S-layers exist in many bacteria, as well as in many archaea. These protein layers serve as scaffolding structures for enzymes, contribute to cell surface adhesion and act as virulence factors, among other functions.
Rights and permissions
About this article
Cite this article
Feltcher, M., Braunstein, M. Emerging themes in SecA2-mediated protein export. Nat Rev Microbiol 10, 779–789 (2012). https://doi.org/10.1038/nrmicro2874
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2874
This article is cited by
-
Plasmepsin V cleaves malaria effector proteins in a distinct endoplasmic reticulum translocation interactome for export to the erythrocyte
Nature Microbiology (2018)
-
The structure of the S-layer of Clostridium difficile
Journal of Cell Communication and Signaling (2018)
-
An alternate mode of oligomerization for E. coli SecA
Scientific Reports (2017)
-
Complete genome sequence of Leuconostoc gelidum subsp. gasicomitatum KG16-1, isolated from vacuum-packaged vegetable sausages
Standards in Genomic Sciences (2016)
-
The pan-genome of Lactobacillus reuteri strains originating from the pig gastrointestinal tract
BMC Genomics (2015)