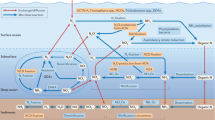
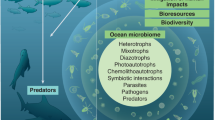
Key Points
-
Microorganisms are the dominant organisms in the sea, both in terms of mass and ecological importance.
-
Most marine microorganisms have not yet been brought into pure cultures in the laboratory, so detailed information about their ecological roles is incomplete.
-
New molecular tools, including gene-based approaches, are beginning to provide data on the diversity and metabolic processes of novel microorganisms that are not yet in culture.
-
The new discipline of microbial oceanography endeavours to observe and understand microbial life in the sea well enough to make accurate ecological predictions of, for example, the impact of climate variations on microbial processes in the sea.
-
Research in microbial oceanography requires an interdisciplinary approach that includes: understanding the physical and chemical habitat properties; making observations over a broad range of time and space scales; designing and implementing hypothesis-testing field experiments; and integrating data into ecosystem-based models. These interdisciplinary interactions must be between scientists who have not traditionally worked together.
-
We are at a unique point in time — one that is characterized by important challenges and great opportunities. New training programmes to prepare the next generation of microbial oceanographers will be essential for continued progress in this important discipline.
Abstract
Life on Earth most likely originated as microorganisms in the sea. Over the past ∼3.5 billion years, microorganisms have shaped and defined Earth's biosphere and have created conditions that have allowed the evolution of macroorganisms and complex biological communities, including human societies. Recent advances in technology have highlighted the vast and previously unknown genetic information that is contained in extant marine microorganisms, from new protein families to novel metabolic processes. Now there is a unique opportunity, using recent advances in molecular ecology, metagenomics, remote sensing of microorganisms and ecological modelling, to achieve a comprehensive understanding of marine microorganisms and their susceptibility to environmental variability and climate change. Contemporary microbial oceanography is truly a sea of opportunity and excitement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Karl, D. M. & Proctor, L. Foundations of microbial oceanography. Oceanography 20, 14–25 (2007).
Duarte, C. M., Gasol, J. M. & Vaque, D. Role of experimental approaches in marine microbial ecology. Aquat. Microb. Ecol. 13, 101–111 (1997).
Doney, S. C., Abbott, M. R., Cullen, J. J., Karl, D. M. & Rothstein, L. From genes to ecosystems: the ocean's new frontier. Frontiers Ecol. Environ. 2, 457–466 (2004). This majestic review is one of the first to establish the importance of environmental genomics as an integral component of marine-ecosystem research.
Rothstein, L. M. et al. Modeling ocean ecosystems: The PARADIGM program. Oceanography 19, 17–45 (2006).
van Leeuwenhoek, A. Concerning little animals by him observed in rain-, well-, sea- and snow-water; as also in water wherein pepper had lain infused. Phil. Trans. Royal Soc. London 12, 821–831 (1677).
Karl, D. M. & Dore, J. E. in Methods in Microbiology Vol. 30 (ed. Paul, J. H.), 13–39 (Academic Press, San Diego, 2001).
Rusch, D. B. et al. The Sorcerer II global ocean sampling expedition: Northwest Atlantic through Eastern Tropical Pacific. PLoS Biol. 5, 0398–0431 (2007). This paper, one of a collection of articles from the recently completed global ocean sampling expedition (see also reference 14), presents an extensive metagenomic data set (6billion base pairs) for surface-water marine microorganisms.
Whitman, W. B., Coleman, D. C. & Wiebe, W. J. Prokaryotes: the unseen majority. Proc. Natl Acad. Sci. USA 95, 6578–6583 (1998).
Hunter-Cevera, J., Karl, D. & Buckley, M. Marine Microbial Diversity: The Key to Earth's Habitability (American Academy of Microbiology, Washington, DC, 2005).
Pace, N. R. A molecular view of microbial diversity and the biosphere. Science 276, 734–740 (1997).
Sogin, M. L. et al. Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc. Natl Acad. Sci. USA 103, 12115–12120 (2006).
Tringe, S. G. & Rubin, E. M. Metagenomics: DNA sequencing of environmental samples. Nature Rev. Genetics 6, 805–814 (2005).
Venter, J. C. et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science 304, 66–74 (2004).
Yooseph, S. et al. The Sorcerer II global ocean sampling expedition: expanding the universe of protein families. PLoS Biol. 5, 0432–0466 (2007).
Ryther, J. H. Potential productivity of the sea. Science 130, 602–608 (1959).
Kolber, Z. Energy cycle in the ocean: powering the microbial world. Oceanography 20, 82–91 (2007).
Cullen, J. J., Franks, P. J. S., Karl, D. M. & Longhurst, A. in The Sea Vol. 12 (eds Robinson, A. R., McCarthy, J. J. & Rothschild, B. J.) 297–336 (John Wiley & Sons, Inc., New York, 2002). A comprehensive review that combines theory, observations and models for the role of physical forcing on the structure and dynamics of marine ecosystems, including microbial biogeochemical processes.
Béjà, O. et al. Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science 289, 1902–1906 (2000). This paper is the first report of proteorhodopsin in the sea, a novel protein that functions as a light-driven proton pump.
Martinez, A., Bradley, A. S., Waldbauer, J. R., Summons, R. E. & DeLong, E. F. Proteorhodopsin photosystem gene expression enables photophosphorylation in a heterologous host. Proc. Natl Acad. Sci. USA 104, 5590–5595 (2007).
Karl, D. M. Hidden in a sea of microbes. Nature 415, 590–591 (2002).
Giovannoni, S. J. et al. Proteorhodopsin in the ubiquitous marine bacterium SAR11. Nature 438, 82–85 (2005).
Gómez-Consarnau, L. et al. Light stimulates growth of proteorhodopsin-containing marine Flavobacteria. Nature 445, 210–213 (2007). This is the first report of proteorhodopsin-based energy capture in a cultivated marine microorganism that shows enhanced cell yield when grown in the light.
del Giorgio, P. A., Cole, J. J. & Cimbleris, A. Respiration rates in bacteria exceed phytoplankton production in unproductive aquatic systems. Nature 385, 148–151 (1997).
Duarte, C. M. & Agusti, S. The CO2 balance of unproductive aquatic ecosystems. Science 281, 234–236 (1998).
Williams, P. J. le B., Morris, P. J. & Karl, D. M. Net community production and metabolic balance at the oligotrophic ocean site, Station ALOHA. Deep-Sea Res. I 51, 1563–1578 (2004).
Karl, D. M., Laws, E. A., Morris, P., Williams, P. J. leB. & Emerson, S. Metabolic balance of the open sea. Nature 426, 32 (2003).
Platt, T. et al. Biological production of the oceans: the case for a consensus. Mar. Ecol. Prog. Ser. 52, 77–88 (1989).
McGowan, J. A. & Hayward, T. L. Mixing and oceanic productivity. Deep-Sea Res. 25, 771–793 (1978).
McGillicuddy, D. J. Jr et al. Influence of mesoscale eddies on new production in the Sargasso Sea. Nature 394, 263–266 (1998).
Uz, B. M., Yoder, J. A. & Osychny, V. Pumping of nutrients to ocean surface waters by the action of propagating planetary waves. Nature 409, 597–600 (2001).
McAndrew, P. M. et al. Metabolic response of oligotrophic plankton communities to deep water nutrient enrichment. Mar. Ecol. Prog. Ser. 332, 63–75 (2007).
Dachs, J. et al. High atmosphere-ocean exchange of organic carbon in the NE subtropical Atlantic. Geophys. Res. Lett. 32, L21807 (2005).
DeLong, E. F. Archaea in coastal marine environments. Proc. Natl Acad. Sci. USA 89, 5685–5689 (1992).
Fuhrman, J. A., McCallum, K. & Davis, A. A. Novel major archaebacterial group from marine plankton. Nature 356, 148–149 (1992).
DeLong, E. F., Wu, K. Y., Prezelin, B. B. & Jovine, R. V. High abundance of Archaea in Antarctic marine picoplankton. Nature 371, 695–697 (1994).
Massana, R., Murray, A. E., Preston, C. M. & DeLong, E. F. Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara channel. Appl. Environ. Microbiol. 63, 50–56 (1997).
Karner, M. B., DeLong, E. F. & Karl, D. M. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409, 507–510 (2001).
DeLong, E. F. Microbial domains in the ocean: a lesson from the Archaea. Oceanography 20, 124–129 (2007).
Karl, D. M., Knauer, G., Martin, J. & Ward, B. Bacterial chemolithotrophy in the ocean is associated with sinking particles. Nature 309, 54–56 (1984).
Pearson, A., McNichol, A. P., Benitez-Nelson, C., Hayes, J. M. & Eglinton, T. I. Origins of lipid biomarkers in Santa Monica Basin surface sediment: a case study using compound-specific 14C analysis. Geochim. Cosmochim. Acta 65, 3123–3137 (2001).
Wuchter, C., Schouten, S., Boschker, H. T. & Sinninghe Damste, J. S. Bicarbonate uptake by marine Crenarchaeota. FEMS Microbiol. Lett. 219, 203–207 (2003).
Könneke, M. et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437, 543–546 (2005). The first paper to report the isolation and cultivation of an ammonia-oxidizing marine archaeon.
Ingalls, A. E. et al. Quantifying archaeal community autotrophy in the mesopelagic ocean using natural radiocarbon. Proc. Natl Acad. Sci. USA 103, 6442–6447 (2006).
Nicol, G. W. & Schleper, C. Ammonia-oxidising Crenarchaeota: important players in the nitrogen cycle? Trends Microbiol. 14, 207–212 (2006).
Wuchter, C. et al. Archaeal nitrification in the ocean. Proc. Natl Acad. Sci. USA 103, 12317–12322 (2006).
Schleper C., Jurgens, G. & Jonuscheit, M. Genomic studies of uncultivated archaea. Nature Rev. Microbiol. 3, 479–488 (2005).
Francis, C. A., Roberts, K. J., Beman, J. M., Santoro, A. E. & Oakley, B. B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl Acad. Sci. USA 102, 14683–14688 (2005).
Mincer, T. J. et al. Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ. Microbiol. 1162–1175 (2007).
Costa, E., Pérez, J. & Kreft, J.-U. Why is metabolic labour divided in nitrification? Trends Microbiol. 14, 213–219 (2006).
Ouverney, C. C. & Fuhrman, J. A. Marine planktonic Archaea take up amino acids. Appl. Environ. Microbiol. 66, 4829–4833 (2000).
Herndl, G. J. et al. Contribution of Archaea to total prokaryotic production in the deep Atlantic Ocean. Appl. Environ. Microbiol. 71, 2303–2309 (2005).
Teira, E., van Aken, H., Veth, C. & Herndl, G. J. Archaeal uptake of enantiomeric amino acids in the meso- and bathypelagic waters of the North Atlantic. Limnol. Oceanogr. 51, 60–69 (2006).
Hallam, S. J. et al. Pathways of carbon assimilation and ammonia oxidation suggested by environmental genomic analyses of marine Crenarchaeota. PLoS Biol. 4, 0520–0536 (2006).
Karl, D. M. A sea of change: biogeochemical variability in the North Pacific Subtropical Gyre. Ecosystems 2, 181–214 (1999).
Michaels, A. F., Karl, D. M. & Capone, D. G. Element stoichiometry, new production and nitrogen fixation. Oceanography 14, 68–77 (2001).
Karl, D. M. et al. in Nitrogen in the Marine Environment (eds Bronk, D., Mulholland, M., Capone, D. & Carpenter, E.) in press (Academic Press).
Karl, D. et al. The role of nitrogen fixation in biogeochemical cycling in the subtropical North Pacific Ocean. Nature 388, 533–538 (1997).
Dore, J. E., Brum, J. R., Tupas, L. M. & Karl, D. M. Seasonal and interannual variability in sources of nitrogen supporting export in the oligotrophic subtropical North Pacific Ocean. Limnol. Oceanogr. 47, 1595–1607 (2002).
Bertilsson, S., Berglund, O., Karl, D. M. & Chisholm, S. W. Elemental composition of marine Prochlorococcus and Synechococcus: implications for the ecological stoichiometry of the sea. Limnol. Oceanogr. 48, 1721–1731 (2003).
White, A., Spitz, Y., Karl, D. M. & Letelier, R. M. Flexible elemental stoichiometry in Trichodesmium spp. and its ecological implications. Limnol. Oceanogr. 51, 1777–1790 (2006).
Sterner, R. W. & Elser, J. J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere (Princeton University Press, Princeton, 2002).
Karl, D. M. in Manual of Environmental Microbiology, Third Edition (eds Hurst, C. J. et al.) (American Society of Microbiology Press, Washington D. C., 2007).
Karl, D. M. Nutrient dynamics in the deep blue sea. Trends Microbiol. 10, 410–418 (2002).
Schindler, D. W. Replication versus realism: the need for ecosystem-scale experiments. Ecosystems 1, 323–334 (1998).
de Baar, H. J. W. et al. Synthesis of iron fertilization experiments: from the Iron Age to the Age of Enlightenment. J. Geophys. Res. 110, C09S16 (2005).
Boyd, P. W. et al. Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions. Science 315, 612–617 (2007). A comprehensive summary and ecological synthesis of the design and implementation of deliberate iron-fertilization experiments in the open sea.
Thingstad, T. F. et al. Nature of phosphorus limitation in the ultraoligotrophic Eastern Mediterranean. Science 309, 1068–1071 (2005).
Berman, T., Walline, P. D., Schneller, A., Rothenberg, J. & Townsend, D. W. Secchi disk depth record: a claim for the Eastern Mediterranean. Limnol. Oceanogr. 30, 447–448 (1985).
Krom, M. D., Kress, N., Brenner, S. & Gordon, L. I. Phosphorus limitation of primary productivity in the Eastern Mediterranean Sea. Limnol. Oceanogr. 36, 424–432 (1991).
Rees, A. P., Law, C. S. & Woodward, E. M. S. High rates of nitrogen fixation during an in-situ phosphate release experiment in the Eastern Mediterranean Sea. Geophys. Res. Lett. 33, L10607 (2006).
Krom, M. D. Preface: CYCLOPS dedicated volume. Deep-Sea Res. II 52, 2877–2878 (2005).
Chisholm, S. W., Falkowski, P. G. & Cullen, J. J. Dis-crediting ocean fertilization. Science 294, 309–310 (2001).
Doney, S. C. The dangers of ocean acidification. Sci. Amer. 294, 58–65 (2006).
ZoBell, C. E. Marine Microbiology: a Monograph on Hydrobacteriology (Chronica Botanica Company, Waltham, 1946).
Wood, E. J. F. Marine Microbial Ecology (Reinhold Publishing Corp., New York, 1965).
Sieburth, J. McN., Smetacek, V. & Lenz, J. Pelagic ecosystem structure: heterotrophic compartments of plankton and their relationship to plankton size fractions. Limnol. Oceanogr. 23, 1256–1263 (1978).
Waterbury, J. B., Watson, S. W., Guillard, R. R. L. & Brand, L. E. Widespread occurrence of a unicellular, marine, planktonic, cyanobacterium. Nature, 277, 293–294 (1979).
Chisholm, S. W., Olson, R. J., Zettler, E. R., Goericke, R., Waterbury, J. B. & Welschmeyer, N. A. A novel free-living prochlorophyte abundant in the oceanic euphotic zone. Nature 334, 340–343 (1988). This paper was the first report of Prochlorococcus in the sea; this and subsequent work have established this microorganism as one of the most important photolithoautotrophs on Earth.
Pace, N. R. Time for a change. Nature 441, 289 (2006).
Longhurst, A. Ecological Geography of the Sea (Academic Press, San Diego, 1998).
DeLong, E. F. et al. Community genomics among stratified microbial assemblages in the ocean's interior. Science 311, 496–503 (2006). A pioneering study that describes genomic variability at Station ALOHA along the depth continuum from the well-lit surface waters to the deep abyss.
Karl, D. M. & Lukas, R. The Hawaii ocean time-series (HOT) program: background, rationale and field implementation. Deep-Sea Res. II 43, 129–156 (1996).
Karl, D. M., Bidigare, R. R. & Letelier, R. M. Long-term changes in plankton community structure and productivity in the North Pacific Subtropical Gyre: the domain shift hypothesis. Deep-Sea Res. II 48, 1449–1470 (2001).
Corno, G. et al. Impact of climate forcing on ecosystem processes in the North Pacific Subtropical Gyre. J. Geophys. Res. 112, C04021 (2007).
Acknowledgements
I thank the HOT and C-MORE program scientists and staff for their important contributions, and the National Science Foundation, the Agouron Institute and the Gordon and Betty Moore Foundation for generous financial support of my research and training endeavors.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Entrez Genome Project
Candidatus Pelagibacter ubique
FURTHER INFORMATION
Glossary
- Phototrophic
-
Able to fix inorganic carbon using energy from light.
- Heterotrophic
-
Able to acquire metabolic energy by the consumption of particulate or dissolved organic matter.
- Microheterotrophs
-
Small (2–20 m) prokaryotic or eukaryotic organisms that are dependent on organic matter.
- Bacterioplankton
-
Bacteria that inhabit the water column of lakes and oceans, either freely suspended or attached to particles.
- Oligotrophic
-
Having low levels of nutrient and algal photosynthetic production (for example, the open ocean).
- Radiative forcing
-
The difference between the incoming radiation energy and the outgoing radiation energy in a given climate system.
- Terrigenous
-
Derived from land or terrestrial ecosystems.
- Pelagic
-
Water-column portion of marine and fresh-water habitats.
- Euphotic zone
-
Upper realms of the oceans (or lakes) that are penetrated by sufficient amounts of light for photosynthetic organisms to grow.
- Primary production
-
Process during which carbon dioxide is incorporated into organic matter by bacteria and algae, using any of a variety of energy sources.
- Mixotrophs
-
Organisms that are part autotrophic and part heterotrophic, such as carnivorous plants.
- Autotrophy
-
The acquisition of metabolic energy from the fixation of inorganic carbon, for example, by photo- or chemosynthesis.
- Picoplankton
-
Organisms that are suspended in the water column that are less than 2 mm in size.
Rights and permissions
About this article
Cite this article
Karl, D. Microbial oceanography: paradigms, processes and promise. Nat Rev Microbiol 5, 759–769 (2007). https://doi.org/10.1038/nrmicro1749
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1749
This article is cited by
-
Depth-Dependent Variables Shape Community Structure and Functionality in the Prince Edward Islands
Microbial Ecology (2021)
-
Mixotrophy in marine picocyanobacteria: use of organic compounds by Prochlorococcus and Synechococcus
The ISME Journal (2020)
-
Inorganic and Organic Carbon Uptake Processes and Their Connection to Microbial Diversity in Meso- and Bathypelagic Arctic Waters (Eastern Fram Strait)
Microbial Ecology (2020)
-
The composition of cultivable bacteria, bacterial pollution, and environmental variables of the coastal areas: an example from the Southeastern Black Sea, Turkey
Environmental Monitoring and Assessment (2020)
-
Ecosystem Structure and Dynamics in the North Pacific Subtropical Gyre: New Views of an Old Ocean
Ecosystems (2017)