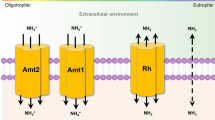
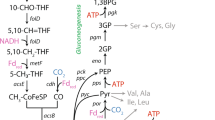
Key Points
-
Nitrogen is an essential component of all living organisms and the main nutrient limiting life on our planet. Its availability depends on diverse nitrogen-transforming reactions that are carried out by microorganisms.
-
Nitrogen-transforming microorganisms are metabolically versatile, rendering their classification as mere nitrifiers, denitrifiers and similar classes inadequate.
-
The classical nitrogen cycle consisting of distinct processes that follow each other in an orderly fashion does not exist. In nature, microorganisms form complex networks that link nitrogen-transforming reactions.
-
Microbial nitrogen-transforming networks both attenuate and exacerbate human-induced global change. They produce and consume the powerful greenhouse gas nitrous oxide, lead to eutrophication of aquatic systems and, at the same time, remove nitrogen from wastewater.
-
There are still many undiscovered nitrogen-transforming reactions that are thermodynamically feasible. The microorganisms catalysing these reactions and the involved biochemical pathways are waiting to be discovered.
Abstract
Nitrogen is an essential component of all living organisms and the main nutrient limiting life on our planet. By far, the largest inventory of freely accessible nitrogen is atmospheric dinitrogen, but most organisms rely on more bioavailable forms of nitrogen, such as ammonium and nitrate, for growth. The availability of these substrates depends on diverse nitrogen-transforming reactions that are carried out by complex networks of metabolically versatile microorganisms. In this Review, we summarize our current understanding of the microbial nitrogen-cycling network, including novel processes, their underlying biochemical pathways, the involved microorganisms, their environmental importance and industrial applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Galloway, J. N. et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892 (2008). A comprehensive overview of the human impact on biogeochemical nitrogen cycling.
Erisman, J. W., Sutton, M. A., Galloway, J., Klimont, Z. & Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639 (2008).
Stein, L. Y. & Klotz, M. G. The nitrogen cycle. Curr. Biol. 26, R94–R98 (2016).
Yan, Y. et al. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc. Natl Acad. Sci. USA 105, 7564–7569 (2008).
Füssel, J. et al. Adaptability as the key to success for the ubiquitous marine nitrite oxidizer Nitrococcus. Sci. Adv. 3, e1700807 (2017).
Daims, H., Lücker, S. & Wagner, M. A. New perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol. 24, 699–712 (2016).
Caranto, J. D. & Lancaster, K. M. Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase. Proc. Natl Acad. Sci. USA 114, 8217–8222 (2017).
Maalcke, W. J. et al. Structural basis of biological NO generation by octaheme oxidoreductases. J. Biol. Chem. 289, 1228–1242 (2014).
Ettwig, K. F. et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464, 543–548 (2010). The discovery of oxygenic denitrification.
Kartal, B. et al. Molecular mechanism of anaerobic ammonium oxidation. Nature 479, 127–130 (2011). Isolation and characterization of the unique enzyme that produces free hydrazine.
Griffin, B. M., Schott, J. & Schink, B. Nitrite, an electron donor for anoxygenic photosynthesis. Science 316, 1870–1870 (2007). The discovery of phototrophic nitrite oxidation.
Daims, H. et al. Complete nitrification by Nitrospira bacteria. Nature 528, 504–509 (2015).
van Kessel, M. A. et al. Complete nitrification by a single microorganism. Nature 528, 555–559 (2015). Together with reference 12, this article reports the discovery of complete nitrification by a single microorganism.
Konneke, M. et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437, 543–546 (2005). The discovery of ammonia-oxidizing archaea.
Risgaard-Petersen, N. et al. Evidence for complete denitrification in a benthic foraminifer. Nature 443, 93–96 (2006).
Thompson, A. W. et al. Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science 337, 1546–1550 (2012). Shows that the ubiquitous unicellular cyanobacterium UCYN-A forms a nitrogen-fixing symbiosis with an algae.
Eady, R. R. Structure-function relationships of alternative nitrogenases. Chem. Rev. 96, 3013–3030 (1996).
Zehr, J. P., Jenkins, B. D., Short, S. M. & Steward, G. F. Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ. Microbiol. 5, 539–554 (2003).
Vitousek, P. M. & Howarth, R. W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 13, 87–115 (1991).
Bothe, H., Schmitz, O., Yates, M. G. & Newton, W. E. Nitrogen Fixation and Hydrogen Metabolism in Cyanobacteria. Microbiol. Mol. Biol. Rev. 74, 529–551 (2010).
Robson, R. L. & Postgate, J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu. Rev. Microbiol. 34, 183–207 (1980).
Berman-Frank, I., Lundgren, P. & Falkowski, P. Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res. Microbiol. 154, 157–164 (2003).
Inomura, K., Bragg, J. & Follows, M. J. A quantitative analysis of the direct and indirect costs of nitrogen fixation: a model based on Azotobacter vinelandii. ISME J. 11, 166–175 (2017).
MacKellar, D. et al. Streptomyces thermoautotrophicus does not fix nitrogen. Sci. Rep. 6, 20086 (2016).
Martinez-Perez, C. et al. The small unicellular diazotrophic symbiont, UCYN-A, is a key player in the marine nitrogen cycle. Nat. Microbiol. 1, 16163 (2016).
Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 12, 168–180 (2014).
Lechene, C. P., Luyten, Y., McMahon, G. & Distel, D. L. Quantitative imaging of nitrogen fixation by individual bacteria within animal cells. Science 317, 1563–1566 (2007).
Burris, R. H. & Roberts, G. Biological nitrogen fixation. Annu. Rev. Nutr. 13, 317–335 (1993).
Hooper, A. B., Vannelli, T., Bergmann, D. J. & Arciero, D. M. Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie Leeuwenhoek 71, 59–67 (1997).
Arp, D. J. & Stein, L. Y. Metabolism of inorganic N compounds by ammonia-oxidizing bacteria. Crit. Rev. Biochem. Mol. Biol. 38, 471–495 (2003).
Prosser, J. I. & Nicol, G. W. Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ. Microbiol. 10, 2931–2941 (2008).
Wuchter, C. et al. Archaeal nitrification in the ocean. Proc. Natl Acad. Sci. USA 103, 12317–12322 (2006).
Francis, C. A., Roberts, K. J., Beman, J. M., Santoro, A. E. & Oakley, B. B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl Acad. Sci. USA 102, 14683–14688 (2005). Shows that ammonia-oxidizing archaea are ubiquitous in the oceans.
Leininger, S. et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442, 806–809 (2006). Shows that archaea are major ammonia oxidizers in soils.
Lehtovirta-Morley, L. E., Stoecker, K., Vilcinskas, A., Prosser, J. I. & Nicol, G. W. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc. Natl Acad. Sci. USA 108, 15892–15897 (2011). The discovery of an acidophilic ammonia oxidizer.
Tourna, M. et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl Acad. Sci. USA 108, 8420–8425 (2011).
Burton, S. A. & Prosser, J. I. Autotrophic ammonia oxidation at low pH through urea hydrolysis. Appl. Environ. Microbiol. 67, 2952–2957 (2001).
Palatinszky, M. et al. Cyanate as an energy source for nitrifiers. Nature 524, 105–108 (2015).
Kits, K. D. et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 549, 269–272 (2017).
Hakemian, A. S. & Rosenzweig, A. C. The biochemistry of methane oxidation. Annu. Rev. Biochem. 76, 223–241 (2007).
Stein, L. Y. & Klotz, M. G. Nitrifying and denitrifying pathways of methanotrophic bacteria. Biochem. Soc. Trans. 39, 1826–1831 (2011).
Stoecker, K. et al. Cohn's Crenothrix is a filamentous methane oxidizer with an unusual methane monooxygenase. Proc. Natl Acad. Sci. USA 103, 2363–2367 (2006).
Oswald, K. et al. Crenothrix are major methane consumers in stratified lakes. ISME J. 11, 2124–2140 (2017).
Simon, J. & Klotz, M. G. Diversity and evolution of bioenergetic systems involved in microbial nitrogen compound transformations. Biochim. Biophys. Acta 1827, 114–135 (2013). An extensive overview of enzymes involved in microbial nitrogen transformations.
Kozlowski, J. A., Stieglmeier, M., Schleper, C., Klotz, M. G. & Stein, L. Y. Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J. 10, 1836–1845 (2016).
Kartal, B. et al. How to make a living from anaerobic ammonium oxidation. FEMS Microbiol. Rev. 37, 428–461 (2013).
Ren, T., Roy, R. & Knowles, R. Production and consumption of nitric oxide by three methanotrophic bacteria. Appl. Environ. Microbiol. 66, 3891–3897 (2000).
Nyerges, G. & Stein, L. Y. Ammonia cometabolism and product inhibition vary considerably among species of methanotrophic bacteria. FEMS Microbiol. Lett. 297, 131–136 (2009).
Schott, J., Griffin, B. M. & Schink, B. Anaerobic phototrophic nitrite oxidation by Thiocapsa sp. strain KS1 and Rhodopseudomonas sp. strain LQ17. Microbiology 156, 2428–2437 (2010).
Strous, M. et al. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440, 790–794 (2006).
Strous, M. et al. Missing lithotroph identified as new planctomycete. Nature 400, 446–449 (1999). The discovery of anaerobic ammonium-oxidizing bacteria.
Koch, H. et al. Growth of nitrite-oxidizing bacteria by aerobic hydrogen oxidation. Science 345, 1052–1054 (2014).
Koch, H. et al. Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus Nitrospira. Proc. Natl Acad. Sci. USA 112, 11371–11376 (2015).
Maia, L. B. & Moura, J. J. How biology handles nitrite. Chem. Rev. 114, 5273–5357 (2014).
Pachiadaki, M. G. et al. Major role of nitrite-oxidizing bacteria in dark ocean carbon fixation. Science 358, 1046–1051 (2017).
Philippot, L., Hallin, S. & Schloter, M. Ecology of denitrifying prokaryotes in agricultural soil. Adv. Agronomy 96, 249–305 (2007).
Lam, P. & Kuypers, M. M. Microbial nitrogen-cycling processes in oxygen minimum zones. Annu. Rev. Mar. Sci. 3, 317–345 (2011).
Kraft, B. et al. The environmental controls that govern the end product of bacterial nitrate respiration. Science 345, 676–679 (2014).
Lundberg, J. O., Weitzberg, E. & Gladwin, M. T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 7, 156–167 (2008).
Moreno-Vivian, C., Cabello, P., Martinez-Luque, M., Blasco, R. & Castillo, F. Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J. Bacteriol. 181, 6573–6584 (1999).
Preisler, A. et al. Biological and chemical sulfide oxidation in a Beggiatoa inhabited marine sediment. ISME J. 1, 341–353 (2007).
Tsementzi, D. et al. SAR11 bacteria linked to ocean anoxia and nitrogen loss. Nature 536, 179–183 (2016).
Lam, P. et al. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc. Natl Acad. Sci. USA 106, 4752–4757 (2009).
Bristow, L. A. et al. N2 production rates limited by nitrite availability in the Bay of Bengal oxygen minimum zone. Nat. Geosci. 10, 24–29 (2017).
Zumft, W. G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61, 533–616 (1997).
Haroon, M. F. et al. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500, 567–570 (2013). The discovery of nitrate-reducing methanotrophic archaea.
Ettwig, K. F. et al. Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc. Natl Acad. Sci. USA 113, 12792–12796 (2016).
Cardoso, R. B. et al. Sulfide oxidation under chemolithoautotrophic denitrifying conditions. Biotechnol. Bioengineer. 95, 1148–1157 (2006).
Weber, K. A., Achenbach, L. A. & Coates, J. D. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 4, 752–764 (2006).
Gruber, N. The marine nitrogen cycle: overview and challenges. Nitrogen Marine Environ. 2, 1–50 (2008).
Stolz, J. F. & Basu, P. Evolution of nitrate reductase: molecular and structural variations on a common function. Chembiochem 3, 198–206 (2002).
Malm, S. et al. The roles of the nitrate reductase NarGHJI, the nitrite reductase NirBD and the response regulator GlnR in nitrate assimilation of Mycobacterium tuberculosis. Microbiology 155, 1332–1339 (2009).
Blöchl, E. et al. Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113 C. Extremophiles 1, 14–21 (1997).
Kamp, A., de Beer, D., Nitsch, J. L., Lavik, G. & Stief, P. Diatoms respire nitrate to survive dark and anoxic conditions. Proc. Natl Acad. Sci. USA 108, 5649–5654 (2011).
Zhou, Z. et al. Ammonia fermentation, a novel anoxic metabolism of nitrate by fungi. J. Biol. Chem. 277, 1892–1896 (2002).
Tikhonova, T. V. et al. Molecular and catalytic properties of a novel cytochrome c nitrite reductase from nitrate-reducing haloalkaliphilic sulfur-oxidizing bacterium Thioalkalivibrio nitratireducens. Biochim. Biophys. Acta 1764, 715–723 (2006).
Atkinson, S. J., Mowat, C. G., Reid, G. A. & Chapman, S. K. An octaheme c-type cytochrome from Shewanella oneidensis can reduce nitrite and hydroxylamine. FEBS Lett. 581, 3805–3808 (2007).
Einsle, O. et al. Structure of cytochrome c nitrite reductase. Nature 400, 476–480 (1999).
Haase, D., Hermann, B., Einsle, O. & Simon, J. Epsilonproteobacterial hydroxylamine oxidoreductase (εHao): characterization of a 'missing link' in the multihaem cytochrome c family. Mol. Microbiol. 105, 127–138 (2017).
Tiedje, J. M. in in Biology of Anaerobic Microorganisms (ed. Zehnder, A. J. B.) 179–244 (Wiley, New York, NY, USA, 1988).
Brunet, R. & Garcia-Gil, L. Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiol. Ecol. 21, 131–138 (1996).
Seitz, H.-J. & Cypionka, H. Chemolithotrophic growth of Desulfovibrio desulfuricans with hydrogen coupled to ammonification of nitrate or nitrite. Arch. Microbiol. 146, 63–67 (1986).
Robertson, E. K., Roberts, K. L., Burdorf, L. D. W., Cook, P. & Thamdrup, B. Dissimilatory nitrate reduction to ammonium coupled to Fe(II) oxidation in sediments of a periodically hypoxic estuary. Limnol. Oceanogr. 61, 365–381 (2016).
Rütting, T., Boeckx, P., Müller, C. & Klemedtsson, L. Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosciences 8, 1779–1791 (2011).
Burgin, A. J. & Hamilton, S. K. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front. Ecol. Environ. 5, 89–96 (2007).
Lomas, M. W. & Lipschultz, F. Forming the primary nitrite maximum: nitrifiers or phytoplankton? Limnol. Oceanogr. 51, 2453–2467 (2006).
Graf, D. R., Jones, C. M. & Hallin, S. Intergenomic comparisons highlight modularity of the denitrification pathway and underpin the importance of community structure for N2O emissions. PloS ONE 9, e114118 (2014).
Bartossek, R., Nicol, G. W., Lanzen, A., Klenk, H. P. & Schleper, C. Homologues of nitrite reductases in ammonia-oxidizing archaea: diversity and genomic context. Environ. Microbiol. 12, 1075–1088 (2010).
Kartal, B. & Keltjens, J. T. Anammox biochemistry: a tale of heme c proteins. Trends Biochem. Sci. 41, 998–1011 (2016).
Fang, F. C. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2, 820–832 (2004).
Hallin, S., Philippot, L., Löffler, F. E., Sanford, R. A. & Jones, C. M. Genomics and ecology of novel N2O-reducing microorganisms. Trends Microbiol. 26, 43–55 (2018).
Ravishankara, A. R., Daniel, J. S. & Portmann, R. W. Nitrous Oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science 326, 123–125 (2009).
Saraiva, L. M., Vicente, J. B. & Teixeira, M. The role of the flavodiiron proteins in microbial nitric oxide detoxification. Adv. Microb. Physiol. 49, 77–129 (2004).
Rodrigues, R. et al. Desulfovibrio gigas flavodiiron protein affords protection against nitrosative stress in vivo. J. Bacteriol. 188, 2745–2751 (2006).
Shoun, H., Fushinobu, S., Jiang, L., Kim, S.-W. & Wakagi, T. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Phil. Trans. R. Soc. B Biol Sci. 367, 1186–1194 (2012).
Wang, J. et al. The roles of the hybrid cluster protein, Hcp, and its reductase, Hcr, in high affinity nitric oxide reduction that protects anaerobic cultures of Escherichia coli against nitrosative stress. Mol. Microbiol. 100, 877–892 (2016).
Hino, T. et al. Structural basis of biological N2O generation by bacterial nitric oxide reductase. Science 330, 1666–1670 (2010).
Matsumoto, Y. et al. Crystal structure of quinol-dependent nitric oxide reductase from Geobacillus stearothermophilus. Nat. Struct. Mol. Biol. 19, 238–245 (2012).
Al-Attar, S. & de Vries, S. An electrogenic nitric oxide reductase. FEBS Lett. 589, 2050–2057 (2015).
Liu, S. et al. Abiotic conversion of extracellular NH2OH contributes to N2O emission during ammonia oxidation. Environ. Sci. Technol. 51, 13122–13132 (2017).
Stocker, T. F. et al. IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge Univ. Press, 2013).
Davidson, E. A. The contribution of manure and fertilizer nitrogen to atmospheric nitrous oxide since 1860. Nat. Geosci. 2, 659–662 (2009).
Crutzen, P., Mosier, A., Smith, K. & Winiwarter, W. N2O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. Atmos. Chem. Phys. 8, 389–395 (2008).
Ettwig, K. F. et al. Bacterial oxygen production in the dark. Front. Microbiol. 3, 273 (2012).
Zumft, W. G. & Kroneck, P. M. Respiratory transformation of nitrous oxide (N2O) to dinitrogen by Bacteria and Archaea. Adv. Microb. Physiol. 52, 107–227 (2006).
Cabello, P., Roldan, M. D. & Moreno-Vivian, C. Nitrate reduction and the nitrogen cycle in archaea. Microbiology 150, 3527–3546 (2004).
Simon, J., Einsle, O., Kroneck, P. M. & Zumft, W. G. The unprecedented nos gene cluster of Wolinella succinogenes encodes a novel respiratory electron transfer pathway to cytochrome c nitrous oxide reductase. FEBS Lett. 569, 7–12 (2004).
Sanford, R. A. et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc. Natl Acad. Sci. USA 109, 19709–19714 (2012).
Jones, C. M. et al. Recently identified microbial guild mediates soil N2O sink capacity. Nat. Climate Change 4, 801–805 (2014).
Piña-Ochoa, E. et al. Widespread occurrence of nitrate storage and denitrification among Foraminifera and Gromiida. Proc. Natl Acad. Sci. USA 107, 1148–1153 (2010).
Philippot, L., Andert, J., Jones, C. M., Bru, D. & Hallin, S. Importance of denitrifiers lacking the genes encoding the nitrous oxide reductase for N2O emissions from soil. Global Change Biol. 17, 1497–1504 (2011).
Codispoti, L. & Christensen, J. Nitrification, denitrification and nitrous oxide cycling in the eastern tropical South Pacific Ocean. Mar. Chem. 16, 277–300 (1985).
Van de Graaf, A. A., de Bruijn, P., Robertson, L. A., Jetten, M. S. & Kuenen, J. G. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142, 2187–2196 (1996).
Mulder, A., Vandegraaf, A. A., Robertson, L. A. & Kuenen, J. G. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 16, 177–183 (1995). The discovery of anaerobic ammonium oxidation.
Dietl, A. et al. The inner workings of the hydrazine synthase multiprotein complex. Nature 527, 394–397 (2015).
Jetten, M. S. M., den Camp, H. J. M. O., Gijs Kuenen, J. & Strous, M. in Bergey's Manual of Systematics of Archaea and Bacteria (ed. Whitman, W. B.) https://doi.org/10.1002/9781118960608.fbm00160 (John Wiley & Sons, Ltd, 2015).
Harhangi, H. R. et al. Hydrazine synthase, a unique phylomarker with which to study the presence and biodiversity of anammox bacteria. Appl. Environ. Microbiol. 78, 752–758 (2012).
Wang, Y. et al. Co-occurrence and distribution of nitrite-dependent anaerobic ammonium and methane-oxidizing bacteria in a paddy soil. Fems Microbiol. Lett. 336, 79–88 (2012).
Maalcke, W. J. et al. Characterization of anammox hydrazine dehydrogenase, a key N2-producing enzyme in the global nitrogen cycle. J. Biol. Chem. 291, 17077–17092 (2016).
Neumann, S. et al. Isolation and characterization of a prokaryotic cell organelle from the anammox bacterium Kuenenia stuttgartiensis. Mol. Microbiol. 94, 794–802 (2014).
de Almeida, N. M. et al. Membrane-bound electron transport systems of an anammox bacterium: a complexome analysis. Biochim. Biophys. Acta 1857, 1694–1704 (2016).
de Almeida, N. M. et al. Immunogold localization of key metabolic enzymes in the anammoxosome and on the tubule-like structures of Kuenenia stuttgartiensis. J. Bacteriol. 197, 2432–2441 (2015).
Kuypers, M. M. et al. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 422, 608–611 (2003). The first detection of anaerobic ammonium-oxidizing bacteria in the environment.
Kuypers, M. M. M. et al. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proc. Natl Acad. Sci. USA 102, 6478–6483 (2005).
Devol, A. H. Nitrogen cycle: Solution to a marine mystery. Nature 422, 575–576 (2003).
Zhu, G. et al. Anaerobic ammonia oxidation in a fertilized paddy soil. ISME J. 5, 1905–1912 (2011).
Bronk, D. A. & Steinberg, D. K. in Nitrogen in the in Marine Environment 2nd edn 385–467 (Academic Press, San Diego, 2008).
Sunagawa, S. et al. Structure and function of the global ocean microbiome. Science 348, 1261359 (2015).
Gruber, N. & Galloway, J. N. An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296 (2008).
Vaksmaa, A. et al. Enrichment of anaerobic nitrate-dependent methanotrophic 'Candidatus Methanoperedens nitroreducens' archaea from an Italian paddy field soil. Appl. Microbiol. Biotechnol. 101, 7075–7084 (2017).
Martens-Habbena, W., Berube, P. M., Urakawa, H., de la Torre, J. R. & Stahl, D. A. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461, 976–979 (2009).
Schleper, C. & Nicol, G. W. Ammonia-oxidising archaea — physiology, ecology and evolution. Adv. Microb. Physiol. 57, 41 (2010).
Pjevac, P. et al. AmoA-targeted polymerase chain reaction primers for the specific detection and quantification of comammox Nitrospira in the environment. Front. Microbiol. 8, 1508 (2017).
Oshiki, M. et al. Nitrate-dependent ferrous iron oxidation by anaerobic ammonium oxidation (anammox) bacteria. Appl. Environ. Microbiol. 79, 4087–4093 (2013).
Ferousi, C. et al. Iron assimilation and utilization in anaerobic ammonium oxidizing bacteria. Curr. Opin. Chem. Biol. 37, 129–136 (2017).
Jensen, M. M. et al. Intensive nitrogen loss over the Omani Shelf due to anammox coupled with dissimilatory nitrite reduction to ammonium. ISME J. 5, 1660–1670 (2011).
Jones, C. M., Graf, D. R. H., Bru, D., Philippot, L. & Hallin, S. The unaccounted yet abundant nitrous oxide-reducing microbial community: a potential nitrous oxide sink. ISME J. 7, 417–426 (2013).
Heylen, K. & Keltjens, J. Redundancy and modularity in membrane-associated dissimilatory nitrate reduction in Bacillus. Front. Microbiol. 3, 371 (2012).
Canfield, D. E., Thamdrup, B. & Kristensen, E. Aquatic Geomicrobiology (Elsevier Academic Press, 2005).
Sutton, M. A. et al. The European Nitrogen Assessment: Sources, Effects and Policy Perspectives (Cambridge Univ. Press, 2011).
Canfield, D. E., Glazer, A. N. & Falkowski, P. G. The evolution and future of Earth's nitrogen cycle. Science 330, 192–196 (2010).
Duce, R. A. et al. Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science 320, 893–897 (2008).
Grosskopf, T. et al. Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488, 361–364 (2012).
McGroddy, M. E., Daufresne, T. & Hedin, L. O. Scaling of C:N:P stoichiometry in forests worldwide: implications of terrestrial redfield-type ratios. Ecology 85, 2390–2401 (2004).
Rittmann, B. E. & McCarty, P. L. Environmental Biotechnology: Principles and Applications (Tata McGraw-Hill Education, 2012).
Kartal, B., Kuenen, J.v. & Van Loosdrecht, M. Sewage treatment with anammox. Science 328, 702–703 (2010).
Lackner, S. et al. Full-scale partial nitritation/anammox experiences — an application survey. Water Res. 55, 292–303 (2014).
Park, H.-D., Wells, G. F., Bae, H., Criddle, C. S. & Francis, C. A. Occurrence of ammonia-oxidizing archaea in wastewater treatment plant bioreactors. Appl. Environ. Microbiol. 72, 5643–5647 (2006).
Luesken, F. A. et al. Simultaneous nitrite-dependent anaerobic methane and ammonium oxidation processes. Appl. Environ. Microbiol. 77, 6802–6807 (2011).
Starkenburg, S. R., Arp, D. J. & Bottomley, P. J. Expression of a putative nitrite reductase and the reversible inhibition of nitrite-dependent respiration by nitric oxide in Nitrobacter winogradskyi Nb-255. Environ. Microbiol. 10, 3036–3042 (2008).
Freitag, A. & Bock, E. Energy conservation in Nitrobacter. FEMS Microbiol. Lett. 66, 157–162 (1990).
Wijma, H. J., Canters, G. W., de Vries, S. & Verbeet, M. P. Bidirectional catalysis by copper-containing nitrite reductase. Biochemistry 43, 10467–10474 (2004).
Sousa, F. L. et al. The superfamily of heme–copper oxygen reductases: types and evolutionary considerations. Biochim. Biophys. Acta 1817, 629–637 (2012).
Rothery, R. A., Workun, G. J. & Weiner, J. H. The prokaryotic complex iron–sulfur molybdoenzyme family. Biochim. Biophys. Acta 1778, 1897–1929 (2008).
Ishii, S., Ikeda, S., Minamisawa, K. & Senoo, K. Nitrogen cycling in rice paddy environments: past achievements and future challenges. Microbes Environ. 26, 282–292 (2011).
Acknowledgements
The authors thank W. Mohr and J. Milucka (Max Planck Institute for Marine Microbiology, Bremen, Germany) for discussions. This work was supported by the Max Planck Society (MPG) and the European Research Council Grant 640422 to B.K.
Author information
Authors and Affiliations
Contributions
M.M.M.K., H.K.M. and B.K. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Reductants
-
The electron-donating compounds in a redox reaction.
- Oxygenic phototrophs
-
Organisms that obtain energy from light and use water as the electron donor, forming molecular oxygen and sugar as products.
- Bacteriocytes
-
Special cells in animals that contain endosymbiotic bacteria.
- Thaumarchaeota
-
The phylum that contains the ammonia-oxidizing archaea.
- Acidophilic
-
The propensity of organisms to grow in acidic environments (pH <6).
- Methanotrophs
-
Organisms that oxidize methane to conserve energy.
- NC10
-
A candidate bacterial phylum, named after the Nullarbor Caves in Australia, that contains 'Candidatus Methylomirabilis oxyfera', which is the first organism discovered that performs methane oxidation coupled to oxygenic denitrification.
- Endergonic
-
A reaction that requires energy input.
- Verrucomicrobia
-
A bacterial phylum with only a few described species, some of which appear to be important in the methane cycle.
- Anoxygenic phototrophs
-
These microorganisms obtain energy from light and use compounds such as hydrogen sulfide instead of water as an electron donor and thus do not produce molecular oxygen.
- Eutrophication
-
An increased input of nutrients that leads to excessive growth of algae or cyanobacteria.
- Proton motive force
-
Proton dislocation creates a difference of charge and pH between two sides of a cell membrane and thereby generates an electrochemical potential, which is used for energy conservation.
- Anaerobic sludge digesters
-
Bioreactors in which excess microbial biomass (sludge) produced during wastewater treatment is anaerobically converted to carbon dioxide, methane, ammonium and reduced sulfur compounds.
- Primary nitrite maxima
-
The peak in nitrite concentrations at the base of the euphotic zone.
- Nitric oxide dismutation
-
Two molecules of nitric oxide are disproportionated into one molecule of molecular oxygen and one molecule of dinitrogen gas.
- Comproportionation
-
A chemical reaction in which two reactants containing the same element with a different oxidation state react to create a product with a single oxidation state.
- Anammoxosome
-
A bacterial organelle found in anaerobic ammonium oxidizing (anammox) bacteria that is the only known prokaryotic membrane-bound structure that is equally divided into daughter cells upon cell division.
- Exergonic
-
A reaction that results in the release of free energy.
- Disproportionation
-
A chemical reaction in which a reactant is split into two species containing the same element with different oxidation states, one more oxidized and the other more reduced than the reactant.
Rights and permissions
About this article
Cite this article
Kuypers, M., Marchant, H. & Kartal, B. The microbial nitrogen-cycling network. Nat Rev Microbiol 16, 263–276 (2018). https://doi.org/10.1038/nrmicro.2018.9
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro.2018.9