Key Points
-
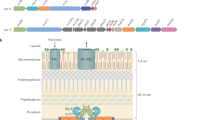
6 kDa early secretory antigenic target (ESAT6) secretion systems (ESX; also known as type VII secretion systems) are sophisticated secretion systems that are present in a wide variety of mycobacterial and non-mycobacterial members of the phylum Actinobacteria.
-
The ESX-1 system of Mycobacterium tuberculosis is the most well-studied ESX system, owing to its important function in virulence, which is linked — at least in part — to the ability of one of its secreted effector proteins, EsxA, to induce phagosomal rupture in host phagocytes.
-
ESX-1 systems are also present in many non-pathogenic, rapid-growing mycobacteria, such as Mycobacterium smegmatis, in which they are involved in conjugal DNA transfer between donor and recipient strains. Interestingly, the ESX-1 effectors EspA, EspC and EspD, which are present in slow-growing, pathogenic mycobacteria, are absent from these non-pathogenic species.
-
Besides ESX-1, M. tuberculosis, which causes tuberculosis, has four additional ESX systems: ESX-3 is involved in iron acquisition; ESX-5 is involved in the secretion of members from two large mycobacterial protein families, named PE and PPE according to their Pro-Glu and Pro-Pro-Glu amino-terminal motifs; and ESX-2 and ESX-4 are systems for which the functions are currently unknown.
-
ESX systems are thought to have evolved from ESX-4 or ESX-4-like systems by gene duplication and diversification, as well as plasmid-mediated horizontal gene transfer.
-
ESX-like systems are secretion systems that are similar to mycobacterial ESX systems but that are found in Gram-positive bacteria in the phylum Firmicutes, rather than in Actinobacteria. Similarly to ESX systems, ESX-like systems contain Esx proteins that have a highly conserved WXG motif, contain an Ftsk–SpoIIIE-like ATPase, and, in some cases (for example, in Staphylococcus aureus), can be involved in pathogenicity.
Abstract
Mycobacterium tuberculosis uses sophisticated secretion systems, named 6 kDa early secretory antigenic target (ESAT6) protein family secretion (ESX) systems (also known as type VII secretion systems), to export a set of effector proteins that helps the pathogen to resist or evade the host immune response. Since the discovery of the esx loci during the M. tuberculosis H37Rv genome project, structural biology, cell biology and evolutionary analyses have advanced our knowledge of the function of these systems. In this Review, we highlight the intriguing roles that these studies have revealed for ESX systems in bacterial survival and pathogenicity during infection with M. tuberculosis. Furthermore, we discuss the diversity of ESX systems that has been described among mycobacteria and selected non-mycobacterial species. Finally, we consider how our knowledge of ESX systems might be applied to the development of novel strategies for the treatment and prevention of disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sorensen, A. L., Nagai, S., Houen, G., Andersen, P. & Andersen, A. B. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63, 1710–1717 (1995).
Brodin, P., Rosenkrands, I., Andersen, P., Cole, S. T. & Brosch, R. ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol. 12, 500–508 (2004).
Gey Van Pittius, N. C. et al. The ESAT-6 gene cluster of Mycobacterium tuberculosis and other high G+C Gram-positive bacteria. Genome Biol. 2, RESEARCH0044 (2001).
Dumas, E. et al. Mycobacterial pan-genome analysis suggests important role of plasmids in the radiation of type VII secretion systems. Genome Biol. Evol. 8, 387–402 (2016). This study provides the first evidence that plasmids seem to have had important roles in the evolution of mycobacterial ESX systems and, consequently, in the pathogenicity of extant mycobacterial pathogens.
Garufi, G., Butler, E. & Missiakas, D. ESAT-6-like protein secretion in Bacillus anthracis. J. Bacteriol. 190, 7004–7011 (2008).
Baptista, C., Barreto, H. C. & Sao-Jose, C. High levels of DegU-P activate an Esat-6-like secretion system in Bacillus subtilis. PLoS ONE. 8, e67840 (2013).
Huppert, L. A. et al. The ESX system in Bacillus subtilis mediates protein secretion. PLoS ONE. 9, e96267 (2014).
Burts, M. L., Williams, W. A., Debord, K. & Missiakas, D. M. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc. Natl Acad. Sci. USA 102, 1169–1174 (2005).
Burts, M. L., DeDent, A. C. & Missiakas, D. M. EsaC substrate for the ESAT-6 secretion pathway and its role in persistent infections of Staphylococcus aureus. Mol. Microbiol. 69, 736–746 (2008).
Way, S. S. & Wilson, C. B. The Mycobacterium tuberculosis ESAT-6 homologue in Listeria monocytogenes is dispensable for growth in vitro and in vivo. Infect. Immun. 73, 6151–6153 (2005).
Houben, E. N., Korotkov, K. V. & Bitter, W. Take five — type VII secretion systems of mycobacteria. Biochim. Biophys. Acta 1844, 1707–1716 (2014).
Daffe, M., Crick, D. C. & Jackson, M. Genetics of capsular polysaccharides and cell envelope (glyco)lipids. Microbiol. Spectr. 2, MGM2-0021-2013 (2014).
Renshaw, P. S. et al. Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. EMBO J. 24, 2491–2498 (2005).
Pallen, M. J. The ESAT-6/WXG100 superfamily — and a new Gram-positive secretion system? Trends Microbiol. 10, 209–212 (2002).
Berthet, F. X., Rasmussen, P. B., Rosenkrands, I., Andersen, P. & Gicquel, B. A. Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144, 3195–3203 (1998).
Cole, S. T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998).
Tekaia, F. et al. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber. Lung Dis. 79, 329–342 (1999).
Newton-Foot, M., Warren, R. M., Sampson, S. L., van Helden, P. D. & Gey van Pittius, N. C. The plasmid-mediated evolution of the mycobacterial ESX (type VII) secretion systems. BMC Evol. Biol. 16, 62 (2016).
Pym, A. S., Brodin, P., Brosch, R., Huerre, M. & Cole, S. T. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 46, 709–717 (2002).
Pym, A. S. et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9, 533–539 (2003).
Hsu, T. et al. The primary mechanism of attenuation of bacillus Calmette–Guérin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl Acad. Sci. USA 100, 12420–12425 (2003).
Lewis, K. N. et al. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette–Guerin attenuation. J. Infect. Dis. 187, 117–123 (2003).
Stanley, S. A., Raghavan, S., Hwang, W. W. & Cox, J. S. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl Acad. Sci. USA 100, 13001–13006 (2003).
Sassetti, C. M. & Rubin, E. J. Genetic requirements for mycobacterial survival during infection. Proc. Natl Acad. Sci. USA 100, 12989–12994 (2003).
Mahairas, G. G., Sabo, P. J., Hickey, M. J., Singh, D. C. & Stover, C. K. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178, 1274–1282 (1996).
Brodin, P. et al. Bacterial artificial chromosome-based comparative genomic analysis identifies Mycobacterium microti as a natural ESAT-6 deletion mutant. Infect. Immun. 70, 5568–5578 (2002).
Serafini, A., Boldrin, F., Palu, G. & Manganelli, R. Characterization of a Mycobacterium tuberculosis ESX-3 conditional mutant: essentiality and rescue by iron and zinc. J. Bacteriol. 191, 6340–6344 (2009).
Siegrist, M. S. et al. Mycobacterial ESX-3 is required for mycobactin-mediated iron acquisition. Proc. Natl Acad. Sci. USA 106, 18792–18797 (2009).
Tufariello, J. M. et al. Separable roles for Mycobacterium tuberculosis ESX-3 effectors in iron acquisition and virulence. Proc. Natl Acad. Sci. USA 113, E348–E357 (2016).
Bottai, D. & Brosch, R. Mycobacterial PE, PPE and ESX clusters: novel insights into the secretion of these most unusual protein families. Mol. Microbiol. 73, 325–328 (2009).
Abdallah, A. M. et al. Mycobacterial secretion systems ESX-1 and ESX-5 play distinct roles in host cell death and inflammasome activation. J. Immunol. 187, 4744–4753 (2011).
Houben, E. N. et al. Composition of the type VII secretion system membrane complex. Mol. Microbiol. 86, 472–484 (2012). A paper that describes the first insights into the components that form the ESX secretion apparatus of ESX systems.
Bottai, D. et al. Disruption of the ESX-5 system of Mycobacterium tuberculosis causes loss of PPE protein secretion, reduction of cell wall integrity and strong attenuation. Mol. Microbiol. 83, 1195–1209 (2012).
Abdallah, A. M. et al. Type VII secretion system of mycobacteria show the way. Nat. Rev. Microbiol. 5, 883–891 (2007).
Bitter, W. et al. Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog. 5, e1000507 (2009).
Desvaux, M., Hebraud, M., Talon, R. & Henderson, I. R. Secretion and subcellular localizations of bacterial proteins: a semantic awareness issue. Trends Microbiol. 17, 139–145 (2009).
Bitter, W., Houben, E. N., Luirink, J. & Appelmelk, B. J. Type VII secretion in mycobacteria: classification in line with cell envelope structure. Trends Microbiol. 17, 337–338 (2009).
Costa, T. R. et al. Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat. Rev. Microbiol. 13, 343–359 (2015).
Gey van Pittius, N. C. et al. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol. Biol. 6, 95 (2006).
Brodin, P. et al. Functional analysis of early secreted antigenic target-6, the dominant T-cell antigen of Mycobacterium tuberculosis, reveals key residues involved in secretion, complex formation, virulence, and immunogenicity. J. Biol. Chem. 280, 33953–33959 (2005).
Poulsen, C., Panjikar, S., Holton, S. J., Wilmanns, M. & Song, Y. H. WXG100 protein superfamily consists of three subfamilies and exhibits an α-helical C-terminal conserved residue pattern. PLoS ONE. 9, e89313 (2014).
Simeone, R. et al. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 8, e1002507 (2012). A paper that confirms and consolidates previous controversial findings on M. tuberculosis -induced phagosomal rupture, using human macrophage-like THP-1 cells and BCG strains that express the M. tuberculosis ESX-1 system.
Houben, D. et al. ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell. Microbiol. 14, 1287–1298 (2012).
Kupz, A. et al. ESAT-6-dependent cytosolic pattern recognition drives noncognate tuberculosis control in vivo. J. Clin. Invest. 126, 2109–2122 (2016). A study that reveals the requirement for phagosomal contact and inflammasome activation for the induction of IL-18-mediated IFNγ production in CD8+ T cells and NK cells.
Champion, P. A., Stanley, S. A., Champion, M. M., Brown, E. J. & Cox, J. S. C-Terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis. Science 313, 1632–1636 (2006).
Daleke, M. H. et al. General secretion signal for the mycobacterial type VII secretion pathway. Proc. Natl Acad. Sci. USA 109, 11342–11347 (2012).
Ates, L. S., Houben, E. N. & Bitter, W. Type VII secretion: a highly versatile secretion system. Microbiol. Spectr. 4, VMBF-0011-2015 (2016).
Strong, M. et al. Toward the structural genomics of complexes: crystal structure of a PE/PPE protein complex from Mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA 103, 8060–8065 (2006).
Brodin, P. et al. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect. Immun. 74, 88–98 (2006).
Chen, J. M. et al. Mycobacterium tuberculosis EspB binds phospholipids and mediates EsxA-independent virulence. Mol. Microbiol. 89, 1154–1166 (2013).
Rosenberg, O. S. et al. Substrates control multimerization and activation of the multi-domain ATPase motor of type VII secretion. Cell 161, 501–512 (2015). A report that provides the first structural insights into the translocation mechanisms in ESX systems, with a focus on the FtsK–SpoIIIE-like ATPase EccC.
de Jonge, M. I. et al. ESAT-6 from Mycobacterium tuberculosis dissociates from its putative chaperone CFP-10 under acidic conditions and exhibits membrane-lysing activity. J. Bacteriol. 189, 6028–6034 (2007).
Peng, X. & Sun, J. Mechanism of ESAT-6 membrane interaction and its roles in pathogenesis of Mycobacterium tuberculosis. Toxicon 116, 29–34 (2016).
Guglielmini, J., de la Cruz, F. & Rocha, E. P. Evolution of conjugation and type IV secretion systems. Mol. Biol. Evol. 30, 315–331 (2013).
Solomonson, M. et al. Structure of the mycosin-1 protease from the mycobacterial ESX-1 protein type VII secretion system. J. Biol. Chem. 288, 17782–17790 (2013).
Ohol, Y. M. et al. Mycobacterium tuberculosis MycP1 protease plays a dual role in regulation of ESX-1 secretion and virulence. Cell Host Microbe 7, 210–220 (2010).
Solomonson, M. et al. Structure of EspB from the ESX-1 type VII secretion system and insights into its export mechanism. Structure 23, 571–583 (2015).
Korotkova, N. et al. Structure of EspB, a secreted substrate of the ESX-1 secretion system of Mycobacterium tuberculosis. J. Struct. Biol. 191, 236–244 (2015).
MacGurn, J. A., Raghavan, S., Stanley, S. A. & Cox, J. S. A non-RD1 gene cluster is required for Snm secretion in Mycobacterium tuberculosis. Mol. Microbiol. 57, 1653–1663 (2005).
Fortune, S. M. et al. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc. Natl Acad. Sci. USA 102, 10676–10681 (2005).
Champion, P. A., Champion, M. M., Manzanillo, P. & Cox, J. S. ESX-1 secreted virulence factors are recognized by multiple cytosolic AAA ATPases in pathogenic mycobacteria. Mol. Microbiol. 73, 950–962 (2009).
McLaughlin, B. et al. A mycobacterium ESX-1-secreted virulence factor with unique requirements for export. PLoS Pathog. 3, e105 (2007).
Bottai, D. et al. ESAT-6 secretion-independent impact of ESX-1 genes espF and espG1 on virulence of Mycobacterium tuberculosis. J. Infect. Dis. 203, 1155–1164 (2011).
Zhang, M. et al. EspI regulates the ESX-1 secretion system in response to ATP levels in Mycobacterium tuberculosis. Mol. Microbiol. 93, 1057–1065 (2014).
Majlessi, L., Prados-Rosales, R., Casadevall, A. & Brosch, R. Release of mycobacterial antigens. Immunol. Rev. 264, 25–45 (2015).
Chen, J. M. et al. EspD is critical for the virulence-mediating ESX-1 secretion system in Mycobacterium tuberculosis. J. Bacteriol. 194, 884–893 (2012).
Ekiert, D. C. & Cox, J. S. Structure of a PE–PPE–EspG complex from Mycobacterium tuberculosis reveals molecular specificity of ESX protein secretion. Proc. Natl Acad. Sci. USA 111, 14758–14763 (2014).
Daleke, M. H. et al. Specific chaperones for the type VII protein secretion pathway. J. Biol. Chem. 287, 31939–31947 (2012).
Korotkova, N. et al. Structure of the Mycobacterium tuberculosis type VII secretion system chaperone EspG5 in complex with PE25–PPE41 dimer. Mol. Microbiol. 94, 367–382 (2014).
Solans, L. et al. A specific polymorphism in Mycobacterium tuberculosis H37Rv causes differential ESAT-6 expression and identifies WhiB6 as a novel ESX-1 component. Infect. Immun. 82, 3446–3456 (2014).
Solans, L. et al. The PhoP-dependent ncRNA Mcr7 modulates the TAT secretion system in Mycobacterium tuberculosis. PLoS Pathog. 10, e1004183 (2014).
Perez, E. et al. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol. Microbiol. 41, 179–187 (2001).
Walters, S. B. et al. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol. Microbiol. 60, 312–330 (2006).
Frigui, W. et al. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog. 4, e33 (2008).
Raghavan, S., Manzanillo, P., Chan, K., Dovey, C. & Cox, J. Secreted transcription factor controls Mycobacterium tuberculosis virulence. Nature 454, 717–721 (2008).
Blasco, B. et al. Virulence regulator EspR of Mycobacterium tuberculosis is a nucleoid-associated protein. PLoS Pathog. 8, e1002621 (2012). A paper that reports the function of EspR to be a nucleoid-associated protein that regulates a large network of genes.
Pang, X. et al. MprAB regulates the espA operon in Mycobacterium tuberculosis and modulates ESX-1 function and host cytokine response. J. Bacteriol. 195, 66–75 (2013).
Hunt, D. M. et al. Long-range transcriptional control of an operon necessary for virulence-critical ESX-1 secretion in Mycobacterium tuberculosis. J. Bacteriol. 194, 2307–2320 (2012).
Gonzalo-Asensio, J. et al. Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc. Natl Acad. Sci. USA 111, 11491–11496 (2014). A study that reveals the role of compensatory mutations in preserving ESX-1 functions in PhoP–PhoR-mutated lineages of the M. tuberculosis complex.
Gordon, S. V. et al. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol. Microbiol. 32, 643–656 (1999).
Brosch, R. et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl Acad. Sci. USA 99, 3684–3689 (2002).
Mostowy, S., Cousins, D., Brinkman, J., Aranaz, A. & Behr, M. A. Genomic deletions suggest a phylogeny for the Mycobacterium tuberculosis complex. J. Infect. Dis. 186, 74–80 (2002).
Gagneux, S. & Small, P. M. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect. Dis. 7, 328–337 (2007).
Boritsch, E. C. et al. A glimpse into the past and predictions for the future: the molecular evolution of the tuberculosis agent. Mol. Microbiol. 93, 835–852 (2014).
Di Luca, M. et al. The ESX-5 associated eccB–eccC locus is essential for Mycobacterium tuberculosis viability. PLoS ONE 7, e52059 (2012).
Ates, L. S. et al. Essential role of the ESX-5 secretion system in outer membrane permeability of pathogenic mycobacteria. PLoS Genet. 11, e1005190 (2015).
Elliott, S. R. & Tischler, A. D. Phosphate starvation: a novel signal that triggers ESX-5 secretion in Mycobacterium tuberculosis. Mol. Microbiol. 100, 510–526 (2016).
Abdallah, A. M. et al. A specific secretion system mediates PPE41 transport in pathogenic mycobacteria. Mol. Microbiol. 62, 667–679 (2006).
Abdallah, A. M. et al. PPE and PE_PGRS proteins of Mycobacterium marinum are transported via the type VII secretion system ESX-5. Mol. Microbiol. 73, 329–340 (2009).
Abdallah, A. M. et al. The ESX-5 secretion system of Mycobacterium marinum modulates the macrophage response. J. Immunol. 181, 7166–7175 (2008).
Daleke, M. H. et al. Conserved Pro-Glu (PE) and Pro-Pro-Glu (PPE) protein domains target LipY lipases of pathogenic mycobacteria to the cell surface via the ESX-5 pathway. J. Biol. Chem. 286, 19024–19034 (2011).
Sassetti, C. M., Boyd, D. H. & Rubin, E. J. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48, 77–84 (2003).
Rodriguez, G. M., Voskuil, M. I., Gold, B., Schoolnik, G. K. & Smith, I. ideR, an essential gene in Mycobacterium tuberculosis: role of IdeR in iron-dependent gene expression, iron metabolism, and oxidative stress response. Infect. Immun. 70, 3371–3381 (2002).
Dubnau, E., Chan, J., Mohan, V. P. & Smith, I. Responses of Mycobacterium tuberculosis to growth in the mouse lung. Infect. Immun. 73, 3754–3757 (2005).
Ilghari, D. et al. Solution structure of the Mycobacterium tuberculosis EsxG–EsxH complex: functional implications and comparisons with other M. tuberculosis Esx family complexes. J. Biol. Chem. 286, 29993–30002 (2011).
Skjot, R. L. et al. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect. Immun. 70, 5446–5453 (2002).
Majlessi, L., Rojas, M. J., Brodin, P. & Leclerc, C. CD8+-T-cell responses of Mycobacterium-infected mice to a newly identified major histocompatibility complex class I-restricted epitope shared by proteins of the ESAT-6 family. Infect. Immun. 71, 7173–7177 (2003).
Hervas-Stubbs, S. et al. High frequency of CD4+ T cells specific for the TB10.4 protein correlates with protection against Mycobacterium tuberculosis infection. Infect. Immun. 74, 3396–3407 (2006).
Mehra, A. et al. Mycobacterium tuberculosis type VII secreted effector EsxH targets host ESCRT to impair trafficking. PLoS Pathog. 9, e1003734 (2013).
Aagaard, C. et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 17, 189–194 (2011).
Feltcher, M. E., Sullivan, J. T. & Braunstein, M. Protein export systems of Mycobacterium tuberculosis: novel targets for drug development? Future Microbiol. 5, 1581–1597 (2010).
Cole, S. T. et al. Massive gene decay in the leprosy bacillus. Nature 409, 1007–1011 (2001).
Singh, P. et al. Insight into the evolution and origin of leprosy bacilli from the genome sequence of Mycobacterium lepromatosis. Proc. Natl Acad. Sci. USA 112, 4459–4464 (2015).
Guinn, K. M. et al. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51, 359–370 (2004).
Sayes, F. et al. Strong immunogenicity and cross-reactivity of Mycobacterium tuberculosis ESX-5 type VII secretion- encoded PE–PPE proteins predicts vaccine potential. Cell Host Microbe 11, 352–363 (2012).
Wards, B. J., de Lisle, G. W. & Collins, D. M. An esat6 knockout mutant of Mycobacterium bovis produced by homologous recombination will contribute to the development of a live tuberculosis vaccine. Tuber. Lung Dis. 80, 185–189 (2000).
Lugo-Villarino, G. & Neyrolles, O. Manipulation of the mononuclear phagocyte system by Mycobacterium tuberculosis. Cold Spring Harb. Perspect. Med. 4, a018549 (2014).
Deretic, V. et al. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cell. Microbiol. 8, 719–727 (2006).
Sturgill-Koszycki, S. et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263, 678–681 (1994).
Stamm, L. M. et al. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J. Exp. Med. 198, 1361–1368 (2003).
van der Wel, N. et al. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129, 1287–1298 (2007). A paper that questions the long-standing view that M. tuberculosis is an entirely intraphagosomal pathogen.
Hart, P. D., Young, M. R., Gordon, A. H. & Sullivan, K. H. Inhibition of phagosome–lysosome fusion in macrophages by certain mycobacteria can be explained by inhibition of lysosomal movements observed after phagocytosis. J. Exp. Med. 166, 933–946 (1987).
Russell, D. G. The ins and outs of the Mycobacterium tuberculosis-containing vacuole. Cell. Microbiol. 18, 1065–1069 (2016).
Simeone, R., Majlessi, L., Enninga, J. & Brosch, R. Perspectives on mycobacterial vacuole-to-cytosol translocation: the importance of cytosolic access. Cell. Microbiol. 18, 1070–1077 (2016).
Simeone, R. et al. Cytosolic access of Mycobacterium tuberculosis: critical impact of phagosomal acidification control and demonstration of occurrence in vivo. PLoS Pathog. 11, e1004650 (2015).
Smith, J. et al. Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect. Immun. 76, 5478–5487 (2008).
Ma, Y., Keil, V. & Sun, J. Characterization of Mycobacterium tuberculosis EsxA membrane insertion: roles of N- and C-terminal flexible arms and central helix–turn–helix motif. J. Biol. Chem. 290, 7314–7322 (2015).
Wassermann, R. et al. Mycobacterium tuberculosis differentially activates cGAS- and inflammasome-dependent intracellular immune responses through ESX-1. Cell Host Microbe. 17, 799–810 (2015).
Watson, R. O. et al. The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe. 17, 811–819 (2015).
Collins, A. C. et al. Cyclic GMP–AMP synthase is an innate immune DNA sensor for Mycobacterium tuberculosis. Cell Host Microbe 17, 820–828 (2015).
Brown, L., Wolf, J. M., Prados-Rosales, R. & Casadevall, A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 13, 620–630 (2015).
Manca, C. et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce TH1 type immunity and is associated with induction of IFN-α/β. Proc. Natl Acad. Sci. USA 98, 5752–5757 (2001).
Stanley, S. A., Johndrow, J. E., Manzanillo, P. & Cox, J. S. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J. Immunol. 178, 3143–3152 (2007).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O'Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Mishra, B. B. et al. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell. Microbiol. 12, 1046–1063 (2010).
Wong, K. W. & Jacobs, W. R. Jr. Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell. Microbiol. 13, 1371–1384 (2011).
Dorhoi, A. et al. Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur. J. Immunol. 42, 374–384 (2012).
Master, S. S. et al. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 3, 224–232 (2008).
Romagnoli, A. et al. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy 8, 1357–1370 (2012).
Davis, J. M. & Ramakrishnan, L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49 (2009).
Stoop, E. J. et al. Zebrafish embryo screen for mycobacterial genes involved in the initiation of granuloma formation reveals a newly identified ESX-1 component. Dis. Model. Mech. 4, 526–536 (2011).
Volkman, H. E. et al. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 327, 466–469 (2010).
Aguilo, J. et al. ESX-1-induced apoptosis is involved in cell-to-cell spread of Mycobacterium tuberculosis. Cell. Microbiol. 15, 1994–2005 (2013).
Weerdenburg, E. M. et al. ESX-5-deficient Mycobacterium marinum is hypervirulent in adult zebrafish. Cell. Microbiol. 14, 728–739 (2012).
Koo, I. C. et al. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell. Microbiol. 10, 1866–1878 (2008).
Ates, L. S. et al. The ESX-5 system of pathogenic mycobacteria is involved in capsule integrity and virulence through its substrate PPE10. PLoS Pathog. 12, e1005696 (2016). A recent paper that reveals the role of the ESX-5 system in mycobacterial capsule production.
Anderson, M., Aly, K. A., Chen, Y. H. & Missiakas, D. Secretion of atypical protein substrates by the ESAT-6 secretion system of Staphylococcus aureus. Mol. Microbiol. 90, 734–743 (2013).
Sundaramoorthy, R., Fyfe, P. K. & Hunter, W. N. Structure of Staphylococcus aureus EsxA suggests a contribution to virulence by action as a transport chaperone and/or adaptor protein. J. Mol. Biol. 383, 603–614 (2008).
Kneuper, H. et al. Heterogeneity in ess transcriptional organization and variable contribution of the Ess/type VII protein secretion system to virulence across closely related Staphylococcus aureus strains. Mol. Microbiol. 93, 928–943 (2014). A paper that provides novel insights into the ESX-like secretion system that is found in strains of Staphylococcus aureus.
Warne, B. et al. The Ess/type VII secretion system of Staphylococcus aureus shows unexpected genetic diversity. BMC Genomics. 17, 222 (2016).
Anderson, M., Chen, Y. H., Butler, E. K. & Missiakas, D. M. EsaD, a secretion factor for the Ess pathway in Staphylococcus aureus. J. Bacteriol. 193, 1583–1589 (2011).
Jager, F., Zoltner, M., Kneuper, H., Hunter, W. N. & Palmer, T. Membrane interactions and self-association of components of the Ess/type VII secretion system of Staphylococcus aureus. FEBS Lett. 590, 349–357 (2016).
Korea, C. G. et al. Staphylococcal Esx proteins modulate apoptosis and release of intracellular Staphylococcus aureus during infection in epithelial cells. Infect. Immun. 82, 4144–4153 (2014).
Ummels, R. et al. Identification of a novel conjugative plasmid in mycobacteria that requires both type IV and type VII secretion. mBio. 5, e01744-14 (2014).
Wang, J. et al. Insights on the emergence of Mycobacterium tuberculosis from the analysis of Mycobacterium kansasii. Genome Biol. Evol. 7, 856–870 (2015).
Kim, B. J. et al. Whole-genome sequence of a novel species, Mycobacterium yongonense DSM 45126T. Genome Announc. 1, e00604-13 (2013).
Gray, T. A., Krywy, J. A., Harold, J., Palumbo, M. J. & Derbyshire, K. M. Distributive conjugal transfer in mycobacteria generates progeny with meiotic-like genome-wide mosaicism, allowing mapping of a mating identity locus. PLoS Biol. 11, e1001602 (2013).
Supply, P. et al. Genomic analysis of smooth tubercle bacilli provides insights into ancestry and pathoadaptation of Mycobacterium tuberculosis. Nat. Genet. 45, 172–179 (2013).
Mortimer, T. D. & Pepperell, C. S. Genomic signatures of distributive conjugal transfer among mycobacteria. Genome Biol. Evol. 6, 2489–2500 (2014).
Boritsch, E. C. et al. pks5-recombination-mediated surface remodelling in Mycobacterium tuberculosis emergence. Nat. Microbiol. 1, 15019 (2016).
Boritsch, E. C. et al. Key experimental evidence of chromosomal DNA transfer among selected tuberculosis-causing mycobacteria. Proc. Natl Acad. Sci. USA 113, 9876–9881 (2016).
Watson, R. O., Manzanillo, P. S. & Cox, J. S. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 150, 803–815 (2012).
Nakagawa, I. et al. Autophagy defends cells against invading group A Streptococcus. Science. 306, 1037–1040 (2004).
Gutierrez, M. G. et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119, 753–766 (2004).
Thurston, T. L., Wandel, M. P., von Muhlinen, N., Foeglein, A. & Randow, F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature. 482, 414–418 (2012).
Zhao, Z. et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 4, 458–469 (2008).
Kimmey, J. M. et al. Unique role for ATG5 in neutrophil-mediated immunopathology during M. tuberculosis infection. Nature 528, 565–569 (2015). A paper that questions the efficacy of autophagy as a major innate defence mechanism against mycobacteria.
Brosch, R. et al. Genome plasticity of BCG and impact on vaccine efficacy. Proc. Natl Acad. Sci. USA 104, 5596–5601 (2007).
Zhang, L. et al. Variable virulence and efficacy of BCG vaccine strains in mice and correlation with genome polymorphisms. Mol. Ther. 24, 398–405 (2016).
Bottai, D. et al. Increased protective efficacy of recombinant BCG strains expressing virulence-neutral proteins of the ESX-1 secretion system. Vaccine. 33, 2710–2718 (2015).
Whole Mycobacteria Cell Vaccines for Tuberculosis Summary Group. Developing whole mycobacteria cell vaccines for tuberculosis: workshop proceedings, Max Planck Institute for Infection Biology, Berlin, Germany, July 9, 2014. Vaccine. 33, 3047–3055 (2015).
Sweeney, K. A. et al. A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat. Med. 17, 1261–1268 (2011).
Sayes, F. et al. CD4+ T cells recognizing PE/PPE antigens directly or via cross reactivity are protective against pulmonary Mycobacterium tuberculosis infection. PLoS Pathog. 12, e1005770 (2016).
Bloemberg, G. V. et al. Acquired resistance to Bedaquiline and Delamanid in therapy for tuberculosis. N. Engl. J. Med. 373, 1986–1988 (2015).
Christophe, T. et al. High content screening identifies decaprenyl-phosphoribose 2′ epimerase as a target for intracellular antimycobacterial inhibitors. PLoS Pathog. 5, e1000645 (2009).
Rybniker, J. et al. Anticytolytic screen identifies inhibitors of mycobacterial virulence protein secretion. Cell Host Microbe 16, 538–548 (2014).
VanderVen, B. C. et al. Novel inhibitors of cholesterol degradation in Mycobacterium tuberculosis reveal how the bacterium's metabolism is constrained by the intracellular environment. PLoS Pathog. 11, e1004679 (2015).
Zuber, B. et al. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacteriol. 190, 5672–5680 (2008).
Hoffmann, C., Leis, A., Niederweis, M., Plitzko, J. M. & Engelhardt, H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl Acad. Sci. USA 105, 3963–3967 (2008).
Poulet, S. & Cole, S. T. Characterisation of the polymorphic GC-rich repetitive sequence (PGRS) present in Mycobacterium tuberculosis. Arch. Microbiol. 163, 87–95 (1995).
Majlessi, L. & Brosch, R. Mycobacterium tuberculosis meets the cytosol: the role of cGAS in anti-mycobacterial immunity. Cell Host Microbe. 17, 733–735 (2015).
Tschopp, J. & Schroder, K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 (2010).
Acknowledgements
The authors gratefully acknowledge the support of grants from the European Community (grant H2020-PHC- 643381), the Agence Nationale de Recherche (grant ANR-14-JAMR-001-02), Institut Pasteur (grant PTR 441) and the Fondation pour la Recherche Médicale (grant DEQ20130326471). R.B. is a member of the LabEx Integrative Biology of Emerging Infectious Diseases (IBEID) consortium at Institut Pasteur. M.I.G. is supported by an M.D.–Ph.D. grant from the University of Groningen, The Netherlands.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Actinobacteria
-
A phylum of Gram-positive bacteria that is characterized by high GC content.
- Mycolic acids
-
Long-chain (C60–C90) fatty acids that are specifically found in the mycobacterial cell envelope; together with extractable lipids, mycolic acids form the mycobacterial outer membrane (also known as the mycomembrane).
- Arabinogalactan–peptidoglycan matrix
-
An essential constituent of the mycobacterial cell wall, consisting of peptidoglycan that is covalently attached to the heteropolysaccharide arabinogalactan, which is linked to the mycolic acid layer.
- Mycobactin
-
An iron-binding compound that is synthesized by most mycobacteria and is necessary for the recovery of iron, which is an essential element for growth.
- Diderm
-
Refers to the presence of two membranes (an inner membrane and an outer membrane) in the cell envelope. Diderm phyla encompass both Gram-negative bacteria and mycobacteria.
- Bootstrap replicates
-
Statistical confidence values in phylogenetic trees that are inferred from phylogenetic bootstrapping, which is an informatics-based method that is based on reconstructing many trees or replicates from minor variations of the input data.
- AAA+ ATPase
-
A member of a large, functionally diverse protein family of ATPases that are associated with various cellular activities that involve energy-dependent remodelling or the translocation of macromolecules.
- Tubercle bacilli
-
Mycobacteria that can cause tuberculosis; that is, the M. tuberculosis complex (MTBC; for example, M. africanum, M. bovis, M. microti and M. tuberculosis) and strains of the M. canettii clade, which represents the putative progenitor pool from which the MTBC evolved.
- Type IV secretion coupling proteins
-
Proteins that recruit nucleotide substrates to the mating pore formation system for subsequent DNA transfer.
- Mycosin 1
-
(MycP1). One of a family of subtilisin-like serine proteases that is found in mycobacteria.
- Pathogenicity-associated genomic island
-
A mobile segment of the genome, transferred by horizontal transfer, that contributes to rapid changes in virulence potential.
- Response regulator
-
A transcriptional regulator that activates transcription of a specific set of genes in response to certain stimuli.
- Two-component system
-
A regulator system that consists of a membrane-embedded sensor protein and a cytoplasmic response regulator. Two-component systems govern numerous cellular activities in diverse species of bacteria and archaea.
- Haemin
-
An iron-containing porphyrin, corresponding to a crystalline chloride of haem, which is obtained when haemoglobin reacts with acetic acid and sodium chloride.
- Pan-genome
-
The full set of genes of a defined species of bacteria or archaea, which comprises all of the genes that are collectively found in individual genomes of the species.
- Pathogen-associated molecular patterns
-
(PAMPs). Conserved molecular structures that are produced by microorganisms and that are recognized as foreign by the receptors of cells of the innate immune system.
- Inflammasome
-
A multiprotein signalling platform that controls the inflammatory response and coordinates antimicrobial host defences through the activation of caspase 1, which induces the processing of pro-interleukin-1β and pro-interleukin-18 into mature cytokines to be released from the cell.
- Granuloma
-
Organized immune cell aggregates that form in response to selected stimuli of an infectious or non-infectious nature.
- Caspase-independent cell death
-
Programmed host cell death that is independent of caspase activation. In the context of mycobacterial infection, caspase-independent cell death suggests the involvement of non-endogenous factors, such as bacterial effectors, in mediating host cell death.
- Severe combined immunodeficiency
-
(SCID). A severe genetic disorder that is characterized by the absence of functioning T lymphocytes and B lymphocytes.
- PE_PGRS proteins
-
Products of a mycobacterial multigene family that is characterized by polymorphic GC-rich repetitive sequence (PGRS) motifs. PE_PGRS proteins have a highly conserved amino-terminal domain of ∼ 110 amino acids, which contains the Pro-Glu (PE) motif, and variable carboxy-terminal domains that contain numerous repetitive sequence motifs.
Rights and permissions
About this article
Cite this article
Gröschel, M., Sayes, F., Simeone, R. et al. ESX secretion systems: mycobacterial evolution to counter host immunity. Nat Rev Microbiol 14, 677–691 (2016). https://doi.org/10.1038/nrmicro.2016.131
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro.2016.131
This article is cited by
-
Exploring virulence in Mycobacterium bovis: clues from comparative genomics and perspectives for the future
Irish Veterinary Journal (2023)
-
Plant commensal type VII secretion system causes iron leakage from roots to promote colonization
Nature Microbiology (2023)
-
Treatments of Mycobacterium tuberculosis and Toxoplasma gondii with Selenium Nanoparticles
BioNanoScience (2023)
-
A lentiviral vector expressing a dendritic cell-targeting multimer induces mucosal anti-mycobacterial CD4+ T-cell immunity
Mucosal Immunology (2022)
-
Novel In Silico mRNA vaccine design exploiting proteins of M. tuberculosis that modulates host immune responses by inducing epigenetic modifications
Scientific Reports (2022)