Key Points
-
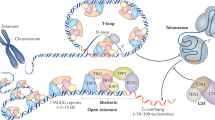
The ends of linear chromosomes pose two problems to eukaryotic cells: the end-protection problem; and the end-replication problem. End protection is necessary to prevent natural ends of chromosomes from being recognized as DNA breaks that require DNA repair, and end replication is required to compensate for the inability of replicative polymerases to copy the extreme ends of chromosomes.
-
The telomerase ribonucleoprotein enzyme uses its internal RNA template for replicating the ends of chromosomes to furnish telomeric DNA, which is bound by telomeric proteins that facilitate chromosome end protection.
-
How does telomerase get recruited to chromosomes? The telomerase–telomere connection is mediated by TPP1 in humans, Ccq1 in fission yeast and Cdc13 in budding yeast. The amino-terminal OB-fold domain of human TPP1 binds telomerase, recruits telomerase to telomeres and increases telomerase processivity.
-
Ccq1, a Schizosaccharomyces pombe-specific telomeric protein, recruits telomerase to telomeres in this organism. Phosphorylation of Ccq1 by the Tel1 and Rad3 kinases regulates its binding to the ever shorter telomeres 1 (Est1) subunit of S. pombe telomerase.
-
Binding of the heterodimeric Ku70–Ku80 to its binding site on Tlc1, the RNA subunit of Saccharomyces cerevisiae telomerase, facilitates localization of telomerase in the nucleus of this organism. The interaction between the telomeric single-stranded DNA-binding protein Cdc13 and the telomerase subunit Est1 tethers budding yeast telomerase to telomeres.
Abstract
Telomeres, the ends of linear eukaryotic chromosomes, are characterized by the presence of multiple repeats of a short DNA sequence. This telomeric DNA is protected from illicit repair by telomere-associated proteins, which in mammals form the shelterin complex. Replicative polymerases are unable to synthesize DNA at the extreme ends of chromosomes, but in unicellular eukaryotes such as yeast and in mammalian germ cells and stem cells, telomere length is maintained by a ribonucleoprotein enzyme known as telomerase. Recent work has provided insights into the mechanisms of telomerase recruitment to telomeres, highlighting the contribution of telomere-associated proteins, including TPP1 in humans, Ccq1 in Schizosaccharomyces pombe and Cdc13 and Ku70–Ku80 in Saccharomyces cerevisiae.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Palm, W. & de Lange, T. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 42, 301–334 (2008).
Levy, M. Z., Allsopp, R. C., Futcher, A. B., Greider, C. W. & Harley, C. B. Telomere end-replication problem and cell aging. J. Mol. Biol. 225, 951–960 (1992).
Pardue, M. L. & DeBaryshe, P. G. Drosophila telomeres: a variation on the telomerase theme. Fly (Austin) 2, 101–110 (2008).
Jaskelioff, M. et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469, 102–106 (2010).
Dokal, I. Dyskeratosis congenita. Hematol. Am. Soc. Hematol. Educ. Program 2011, 480–486 (2011).
Cifuentes-Rojas, C. & Shippen, D. E. Telomerase regulation. Mutat. Res. 730, 20–27 (2012).
Lewis, K. A. & Wuttke, D. S. Telomerase and telomere-associated proteins: structural insights into mechanism and evolution. Structure 20, 28–39 (2012).
Egan, E. D. & Collins, K. Biogenesis of telomerase ribonucleoproteins. RNA 18, 1747–1759 (2012). This excellent review describes the T. thermophila telomerase holoenzyme, which is not covered in detail in this Review.
Zakian, V. A. Telomeres: the beginnings and ends of eukaryotic chromosomes. Exp. Cell Res. 318, 1456–1460 (2012).
Williamson, J. R., Raghuraman, M. K. & Cech, T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell 59, 871–880 (1989).
Sundquist, W. I. & Klug, A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature 342, 825–829 (1989).
Griffith, J. D. et al. Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 (1999).
Fairall, L., Chapman, L., Moss, H., de Lange, T. & Rhodes, D. Structure of the TRFH dimerization domain of the human telomeric proteins TRF1 and TRF2. Mol. Cell 8, 351–361 (2001).
Court, R., Chapman, L., Fairall, L. & Rhodes, D. How the human telomeric proteins TRF1 and TRF2 recognize telomeric DNA: a view from high-resolution crystal structures. EMBO Rep. 6, 39–45 (2005).
Nishikawa, T. et al. Solution structure of a telomeric DNA complex of human TRF1. Structure 9, 1237–1251 (2001).
Hanaoka, S., Nagadoi, A. & Nishimura, Y. Comparison between TRF2 and TRF1 of their telomeric DNA-bound structures and DNA-binding activities. Protein Sci. 14, 119–130 (2005).
Cooper, J. P., Nimmo, E. R., Allshire, R. C. & Cech, T. R. Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature 385, 744–747 (1997).
Shore, D. & Nasmyth, K. Purification and cloning of a DNA binding protein from yeast that binds to both silencer and activator elements. Cell 51, 721–732 (1987).
Hardy, C. F., Sussel, L. & Shore, D. A. RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 6, 801–814 (1992).
Wotton, D. & Shore, D. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 11, 748–760 (1997).
Li, B., Oestreich, S. & de Lange, T. Identification of human Rap1: implications for telomere evolution. Cell 101, 471–483 (2000).
Chikashige, Y. & Hiraoka, Y. Telomere binding of the Rap1 protein is required for meiosis in fission yeast. Curr. Biol. 11, 1618–1623 (2001).
Horvath, M. P., Schweiker, V. L., Bevilacqua, J. M., Ruggles, J. A. & Schultz, S. C. Crystal structure of the Oxytricha nova telomere end binding protein complexed with single strand DNA. Cell 95, 963–974 (1998).
Baumann, P. & Cech, T. R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292, 1171–1175 (2001).
Baumann, P., Podell, E. & Cech, T. R. Human Pot1 (protection of telomeres) protein: cytolocalization, gene structure, and alternative splicing. Mol. Cell. Biol. 22, 8079–8087 (2002).
Loayza, D. & De Lange, T. POT1 as a terminal transducer of TRF1 telomere length control. Nature 423, 1013–1018 (2003).
Lei, M., Podell, E. R. & Cech, T. R. Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nature Struct. Mol. Biol. 11, 1223–1229 (2004).
Hockemeyer, D., Daniels, J. P., Takai, H. & de Lange, T. Recent expansion of the telomeric complex in rodents: two distinct POT1 proteins protect mouse telomeres. Cell 126, 63–77 (2006).
Wu, L. et al. Pot1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell 126, 49–62 (2006).
Wang, F. et al. The POT1–TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 (2007). Reports the stimulation of telomerase in vitro by the POT1–TPP1 complex and also solves the high-resolution crystal structure of the N-terminal OB-fold domain of TPP1.
Hockemeyer, D. et al. Telomere protection by mammalian Pot1 requires interaction with Tpp1. Nature Struct. Mol. Biol. 14, 754–761 (2007).
Guo, X. et al. Dysfunctional telomeres activate an ATM–ATR-dependent DNA damage response to suppress tumorigenesis. EMBO J. 26, 4709–4719 (2007).
Liu, D. et al. PTOP interacts with POT1 and regulates its localization to telomeres. Nature Cell Biol. 6, 673–680 (2004).
Denchi, E. L. & de Lange, T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448, 1068–1071 (2007).
Taylor, D. J., Podell, E. R., Taatjes, D. J. & Cech, T. R. Multiple POT1–TPP1 proteins coat and compact long telomeric single-stranded DNA. J. Mol. Biol. 410, 10–17 (2011).
Lei, M., Podell, E. R., Baumann, P. & Cech, T. R. DNA self-recognition in the structure of Pot1 bound to telomeric single-stranded DNA. Nature 426, 198–203 (2003).
Miyoshi, T., Kanoh, J., Saito, M. & Ishikawa, F. Fission yeast Pot1–Tpp1 protects telomeres and regulates telomere length. Science 320, 1341–1344 (2008). Identifies the S. pombe telomeric proteins Ccq1, Poz1 and Tpz1 and demonstrates that Ccq1 is a positive regulator of telomere length.
Nandakumar, J. & Cech, T. R. DNA-induced dimerization of the single-stranded DNA binding telomeric protein Pot1 from Schizosaccharomyces pombe. Nucleic Acids Res. 40, 235–244 (2011).
Altschuler, S. E., Dickey, T. H. & Wuttke, D. S. Schizosaccharomyces pombe protection of telomeres 1 utilizes alternate binding modes to accommodate different telomeric sequences. Biochemistry 50, 7503–7513 (2011).
Nugent, C. I., Hughes, T. R., Lue, N. F. & Lundblad, V. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science 274, 249–252 (1996).
Grandin, N., Reed, S. I. & Charbonneau, M. Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes Dev. 11, 512–527 (1997).
Grandin, N., Damon, C. & Charbonneau, M. Ten1 functions in telomere end protection and length regulation in association with Stn1 and Cdc13. EMBO J. 20, 1173–1183 (2001).
Anderson, E. M., Halsey, W. A. & Wuttke, D. S. Delineation of the high-affinity single-stranded telomeric DNA-binding domain of Saccharomyces cerevisiae Cdc13. Nucleic Acids Res. 30, 4305–4313 (2002).
Mitton-Fry, R. M., Anderson, E. M., Hughes, T. R., Lundblad, V. & Wuttke, D. S. Conserved structure for single-stranded telomeric DNA recognition. Science 296, 145–147 (2002).
Mitton-Fry, R. M., Anderson, E. M., Theobald, D. L., Glustrom, L. W. & Wuttke, D. S. Structural basis for telomeric single-stranded DNA recognition by yeast Cdc13. J. Mol. Biol. 338, 241–255 (2004).
Sun, J. et al. Structural bases of dimerization of yeast telomere protein Cdc13 and its interaction with the catalytic subunit of DNA polymerase α. Cell Res. 21, 258–274 (2011).
Gao, H., Cervantes, R. B., Mandell, E. K., Otero, J. H. & Lundblad, V. RPA-like proteins mediate yeast telomere function. Nature Struct. Mol. Biol. 14, 208–214 (2007).
Kim, S. H., Kaminker, P. & Campisi, J. TIN2, a new regulator of telomere length in human cells. Nature Genet. 23, 405–412 (1999).
Kim, S. H. et al. TIN2 mediates functions of TRF2 at human telomeres. J. Biol. Chem. 279, 43799–43804 (2004).
Ye, J. Z. et al. TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J. Biol. Chem. 279, 47264–47271 (2004).
Ye, J. Z. et al. POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev. 18, 1649–1654 (2004).
Houghtaling, B. R., Cuttonaro, L., Chang, W. & Smith, S. A dynamic molecular link between the telomere length regulator TRF1 and the chromosome end protector TRF2. Curr. Biol. 14, 1621–1631 (2004).
Chen, Y. et al. A shared docking motif in TRF1 and TRF2 used for differential recruitment of telomeric proteins. Science 319, 1092–1096 (2008).
O'Connor, M. S., Safari, A., Xin, H., Liu, D. & Songyang, Z. A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc. Natl Acad. Sci. USA 103, 11874–11879 (2006).
Liu, D., O'Connor, M. S., Qin, J. & Songyang, Z. Telosome, a mammalian telomere-associated complex formed by multiple telomeric proteins. J. Biol. Chem. 279, 51338–51342 (2004).
Takai, K. K., Hooper, S. M., Blackwood, S. L., Gandhi, R. & de Lange, T. In vivo stoichiometry of shelterin components. J. Biol. Chem. 285, 1457–1467 (2009).
Sfeir, A. & de Lange, T. Removal of shelterin reveals the telomere end-protection problem. Science 336, 593–597 (2012). By conditional deletion of the entire mouse shelterin complex, the authors reveal six DNA damage signalling pathways that are normally repressed at telomeres.
Greider, C. W. & Blackburn, E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature 337, 331–337 (1989).
Blasco, M. A., Funk, W., Villeponteau, B. & Greider, C. W. Functional characterization and developmental regulation of mouse telomerase RNA. Science 269, 1267–1270 (1995).
Singer, M. S. & Gottschling, D. E. TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science 266, 404–409 (1994).
Webb, C. J. & Zakian, V. A. Identification and characterization of the Schizosaccharomyces pombe TER1 telomerase RNA. Nature Struct. Mol. Biol. 15, 34–42 (2008).
Leonardi, J., Box, J. A., Bunch, J. T. & Baumann, P. TER1, the RNA subunit of fission yeast telomerase. Nature Struct. Mol. Biol. 15, 26–33 (2008).
Chen, J. L. & Greider, C. W. Template boundary definition in mammalian telomerase. Genes Dev. 17, 2747–2752 (2003).
Tzfati, Y., Fulton, T. B., Roy, J. & Blackburn, E. H. Template boundary in a yeast telomerase specified by RNA structure. Science 288, 863–867 (2000).
Box, J. A., Bunch, J. T., Zappulla, D. C., Glynn, E. F. & Baumann, P. A flexible template boundary element in the RNA subunit of fission yeast telomerase. J. Biol. Chem. 283, 24224–24233 (2008).
Theimer, C. A., Blois, C. A. & Feigon, J. Structure of the human telomerase RNA pseudoknot reveals conserved tertiary interactions essential for function. Mol. Cell 17, 671–682 (2005).
Shefer, K. et al. A triple helix within a pseudoknot is a conserved and essential element of telomerase RNA. Mol. Cell. Biol. 27, 2130–2143 (2007).
Qiao, F. & Cech, T. R. Triple-helix structure in telomerase RNA contributes to catalysis. Nature Struct. Mol. Biol. 15, 634–640 (2008).
Chen, J. L. & Greider, C. W. Determinants in mammalian telomerase RNA that mediate enzyme processivity and cross-species incompatibility. EMBO J. 22, 304–314 (2003).
Lai, C. K., Miller, M. C. & Collins, K. Roles for RNA in telomerase nucleotide and repeat addition processivity. Mol. Cell 11, 1673–1683 (2003).
Berman, A. J., Akiyama, B. M., Stone, M. D. & Cech, T. R. The RNA accordion model for template positioning by telomerase RNA during telomeric DNA synthesis. Nature Struct. Mol. Biol. 18, 1371–1375 (2011).
Qi, X. et al. RNA/DNA hybrid binding affinity determines telomerase template-translocation efficiency. EMBO J. 31, 150–161 (2011).
Bley, C. J. et al. RNA–protein binding interface in the telomerase ribonucleoprotein. Proc. Natl Acad. Sci. USA 108, 20333–20338 (2011).
Chen, J. L., Blasco, M. A. & Greider, C. W. Secondary structure of vertebrate telomerase RNA. Cell 100, 503–514 (2000).
Mitchell, J. R. & Collins, K. Human telomerase activation requires two independent interactions between telomerase RNA and telomerase reverse transcriptase. Mol. Cell 6, 361–371 (2000).
Chen, J. L., Opperman, K. K. & Greider, C. W. A critical stem-loop structure in the CR4-CR5 domain of mammalian telomerase RNA. Nucleic Acids Res. 30, 592–597 (2002).
Mitchell, J. R., Wood, E. & Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 402, 551–555 (1999).
Venteicher, A. S. et al. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science 323, 644–648 (2009).
Tycowski, K. T., Shu, M. D., Kukoyi, A. & Steitz, J. A. A conserved WD40 protein binds the Cajal body localization signal of scaRNP particles. Mol. Cell 34, 47–57 (2009).
Seto, A. G., Livengood, A. J., Tzfati, Y., Blackburn, E. H. & Cech, T. R. A bulged stem tethers Est1p to telomerase RNA in budding yeast. Genes Dev. 16, 2800–2812 (2002).
Peterson, S. E. et al. The function of a stem–loop in telomerase RNA is linked to the DNA repair protein Ku. Nature Genet. 27, 64–67 (2001).
Stellwagen, A. E., Haimberger, Z. W., Veatch, J. R. & Gottschling, D. E. Ku interacts with telomerase RNA to promote telomere addition at native and broken chromosome ends. Genes Dev. 17, 2384–2395 (2003).
Seto, A. G., Zaug, A. J., Sobel, S. G., Wolin, S. L. & Cech, T. R. Saccharomyces cerevisiae telomerase is an Sm small nuclear ribonucleoprotein particle. Nature 401, 177–180 (1999).
Box, J. A., Bunch, J. T., Tang, W. & Baumann, P. Spliceosomal cleavage generates the 3′ end of telomerase RNA. Nature 456, 910–914 (2008).
Tang, W., Kannan, R., Blanchette, M. & Baumann, P. Telomerase RNA biogenesis involves sequential binding by Sm and Lsm complexes. Nature 484, 260–264 (2012).
Fu, D. & Collins, K. Purification of human telomerase complexes identifies factors involved in telomerase biogenesis and telomere length regulation. Mol. Cell 28, 773–785 (2007).
Zappulla, D. C. & Cech, T. R. Yeast telomerase RNA: a flexible scaffold for protein subunits. Proc. Natl Acad. Sci. USA 101, 10024–10029 (2004).
Zappulla, D. C., Goodrich, K. & Cech, T. R. A miniature yeast telomerase RNA functions in vivo and reconstitutes activity in vitro. Nature Struct. Mol. Biol. 12, 1072–1077 (2005).
Lebo, K. J. & Zappulla, D. C. Stiffened yeast telomerase RNA supports RNP function in vitro and in vivo. RNA 18, 1666–1678 (2012).
Lingner, J. et al. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science 276, 561–567 (1997).
Meyerson, M. et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90, 785–795 (1997).
Nakamura, T. M. et al. Telomerase catalytic subunit homologs from fission yeast and human. Science 277, 955–959 (1997).
Gillis, A. J., Schuller, A. P. & Skordalakes, E. Structure of the Tribolium castaneum telomerase catalytic subunit TERT. Nature 455, 633–637 (2008).
Mitchell, M., Gillis, A., Futahashi, M., Fujiwara, H. & Skordalakes, E. Structural basis for telomerase catalytic subunit TERT binding to RNA template and telomeric DNA. Nature Struct. Mol. Biol. 17, 513–518 (2010).
Lue, N. F. A physical and functional constituent of telomerase anchor site. J. Biol. Chem. 280, 26586–26591 (2005).
Moriarty, T. J., Ward, R. J., Taboski, M. A. & Autexier, C. An anchor site-type defect in human telomerase that disrupts telomere length maintenance and cellular immortalization. Mol. Biol. Cell 16, 3152–3161 (2005).
Finger, S. N. & Bryan, T. M. Multiple DNA-binding sites in Tetrahymena telomerase. Nucleic Acids Res. 36, 1260–1272 (2008).
Romi, E. et al. High-resolution physical and functional mapping of the template adjacent DNA binding site in catalytically active telomerase. Proc. Natl Acad. Sci. USA 104, 8791–8796 (2007).
Wyatt, H. D., Tsang, A. R., Lobb, D. A. & Beattie, T. L. Human telomerase reverse transcriptase (hTERT) Q169 is essential for telomerase function in vitro and in vivo. PLoS ONE 4, e7176 (2009).
Jurczyluk, J. et al. Direct involvement of the TEN domain at the active site of human telomerase. Nucleic Acids Res. 39, 1774–1788 (2011).
Armbruster, B. N., Banik, S. S., Guo, C., Smith, A. C. & Counter, C. M. N-terminal domains of the human telomerase catalytic subunit required for enzyme activity in vivo. Mol. Cell. Biol. 21, 7775–7786 (2001).
Talley, J. M., DeZwaan, D. C., Maness, L. D., Freeman, B. C. & Friedman, K. L. Stimulation of yeast telomerase activity by the ever shorter telomere 3 (Est3) subunit is dependent on direct interaction with the catalytic protein Est2. J. Biol. Chem. 286, 26431–26439 (2011).
van Steensel, B. & de Lange, T. Control of telomere length by the human telomeric protein TRF1. Nature 385, 740–743 (1997).
Ye, J. Z. & de Lange, T. TIN2 is a tankyrase 1 PARP modulator in the TRF1 telomere length control complex. Nature Genet. 36, 618–623 (2004).
Xin, H. et al. TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature 445, 559–562 (2007). Demonstrates that the N-terminal OB-fold domain of TPP1 binds telomerase.
Latrick, C. M. & Cech, T. R. POT1–TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation. EMBO J. 29, 924–933 (2010).
Abreu, E. et al. TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol. Cell. Biol. 30, 2971–2982 (2010). Reports the requirement of the N-terminal OB-fold domain of TPP1 in telomerase recruitment to human telomeres.
Tejera, A. M. et al. TPP1 is required for TERT recruitment, telomere elongation during nuclear reprogramming, and normal skin development in mice. Dev. Cell 18, 775–789 (2010). Describes the role of TPP1 in telomerase recruitment to mouse telomeres.
Zhong, F. L. et al. TPP1 OB-fold domain controls telomere maintenance by recruiting telomerase to chromosome ends. Cell 150, 481–494 (2012). Reports the sufficiency of the N-terminal OB-fold domain of TPP1 in telomerase recruitment to human telomeres and identifies key amino acids on TPP1 and TERT that are required for telomerase recruitment.
Sexton, A. N., Youmans, D. T. & Collins, K. Specificity requirements for human telomere protein interaction with telomerase holoenzyme. J. Biol. Chem. 287, 34455–34464 (2012).
Nandakumar, J. et al. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature 492, 285–289 (2012). Identifies a patch of amino acids on the surface of the N-terminal OB-fold domain of TPP1 that mediates telomerase binding, telomerase recruitment, telomerase processivity stimulation and telomere lengthening.
Tomlinson, R. L., Ziegler, T. D., Supakorndej, T., Terns, R. M. & Terns, M. P. Cell cycle-regulated trafficking of human telomerase to telomeres. Mol. Biol. Cell 17, 955–965 (2006).
Jady, B. E., Richard, P., Bertrand, E. & Kiss, T. Cell cycle-dependent recruitment of telomerase RNA and Cajal bodies to human telomeres. Mol. Biol. Cell 17, 944–954 (2006).
Lei, M., Zaug, A. J., Podell, E. R. & Cech, T. R. Switching human telomerase on and off with hPOT1 protein in vitro. J. Biol. Chem. 280, 20449–20456 (2005).
Zaug, A. J., Podell, E. R., Nandakumar, J. & Cech, T. R. Functional interaction between telomere protein TPP1 and telomerase. Genes Dev. 24, 613–622 (2010).
Zhao, Y. et al. Processive and distributive extension of human telomeres by telomerase under homeostatic and nonequilibrium conditions. Mol. Cell 42, 297–307 (2011).
Savage, S. A. et al. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am. J. Hum. Genet. 82, 501–509 (2008).
Walne, A. J., Vulliamy, T., Beswick, R., Kirwan, M. & Dokal, I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood 112, 3594–3600 (2008).
Sasa, G. S., Ribes-Zamora, A., Nelson, N. D. & Bertuch, A. A. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clin. Genet. 81, 470–478 (2012).
Bessler, M., Wilson, D. B. & Mason, P. J. Dyskeratosis congenita. FEBS Lett. 584, 3831–3838 (2010).
Yang, D., He, Q., Kim, H., Ma, W. & Songyang, Z. TIN2 protein dyskeratosis congenita missense mutants are defective in association with telomerase. J. Biol. Chem. 286, 23022–23030 (2011).
Canudas, S. et al. A role for heterochromatin protein 1γ at human telomeres. Genes Dev. 25, 1807–1819 (2011).
Houghtaling, B. R., Canudas, S. & Smith, S. A role for sister telomere cohesion in telomere elongation by telomerase. Cell Cycle 11, 19–25 (2012).
Wenz, C. et al. Human telomerase contains two cooperating telomerase RNA molecules. EMBO J. 20, 3526–3534 (2001).
Cristofari, G. & Lingner, J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J. 25, 565–574 (2006).
Prescott, J. & Blackburn, E. H. Functionally interacting telomerase RNAs in the yeast telomerase complex. Genes Dev. 11, 2790–2800 (1997).
Moser, B. A., Chang, Y. T., Kosti, J. & Nakamura, T. M. Tel1ATM and Rad3ATR kinases promote Ccq1–Est1 interaction to maintain telomeres in fission yeast. Nature Struct. Mol. Biol. 18, 1408–1413 (2011).
Yamazaki, H., Tarumoto, Y. & Ishikawa, F. Tel1ATM and Rad3ATR phosphorylate the telomere protein Ccq1 to recruit telomerase and elongate telomeres in fission yeast. Genes Dev. 26, 241–246 (2012). References 127 and 128 demonstrate how the ATM and ATR kinases of S. pombe , Tel1 and Rad3, phosphorylate Ccq1 to facilitate Est1 binding and telomerase recruitment.
Verdun, R. E. & Karlseder, J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell 127, 709–720 (2006).
Sabourin, M. & Zakian, V. A. ATM-like kinases and regulation of telomerase: lessons from yeast and mammals. Trends Cell Biol. 18, 337–346 (2008).
Moser, B. A. et al. Differential arrival of leading and lagging strand DNA polymerases at fission yeast telomeres. EMBO J. 28, 810–820 (2009).
Moser, B. A., Subramanian, L., Khair, L., Chang, Y. T. & Nakamura, T. M. Fission yeast Tel1ATM and Rad3ATR promote telomere protection and telomerase recruitment. PLoS Genet. 5, e1000622 (2009).
Naito, T., Matsuura, A. & Ishikawa, F. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nature Genet. 20, 203–206 (1998).
Webb, C. J. & Zakian, V. A. Schizosaccharomyces pombe Ccq1 and TER1 bind the 14-3-3-like domain of Est1, which promotes and stabilizes telomerase–telomere association. Genes Dev. 26, 82–91 (2012).
Lundblad, V. & Szostak, J. W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell 57, 633–643 (1989).
Lendvay, T. S., Morris, D. K., Sah, J., Balasubramanian, B. & Lundblad, V. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics 144, 1399–1412 (1996).
Hughes, T. R., Evans, S. K., Weilbaecher, R. G. & Lundblad, V. The Est3 protein is a subunit of yeast telomerase. Curr. Biol. 10, 809–812 (2000).
Virta-Pearlman, V., Morris, D. K. & Lundblad, V. Est1 has the properties of a single-stranded telomere end-binding protein. Genes Dev. 10, 3094–3104 (1996).
Qi, H. & Zakian, V. A. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase α and the telomerase-associated est1 protein. Genes Dev. 14, 1777–1788 (2000).
Zhou, J., Hidaka, K. & Futcher, B. The Est1 subunit of yeast telomerase binds the Tlc1 telomerase RNA. Mol. Cell. Biol. 20, 1947–1955 (2000).
Evans, S. K. & Lundblad, V. Est1 and Cdc13 as comediators of telomerase access. Science 286, 117–120 (1999). Shows that Est1 can bridge Est2–Tlc1 and Cdc13 at telomeres by demonstrating that the requirement of Est1 for telomerase recruitment can be waived if the Est2 polypeptide is fused with the Cdc13 protein.
Pennock, E., Buckley, K. & Lundblad, V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell 104, 387–396 (2001).
Wu, Y. & Zakian, V. A. The telomeric Cdc13 protein interacts directly with the telomerase subunit Est1 to bring it to telomeric DNA ends in vitro. Proc. Natl Acad. Sci. USA 108, 20362–20369 (2011).
Bianchi, A., Negrini, S. & Shore, D. Delivery of yeast telomerase to a DNA break depends on the recruitment functions of Cdc13 and Est1. Mol. Cell 16, 139–146 (2004).
Chan, A., Boule, J. B. & Zakian, V. A. Two pathways recruit telomerase to Saccharomyces cerevisiae telomeres. PLoS Genet. 4, e1000236 (2008).
Tseng, S. F., Shen, Z. J., Tsai, H. J., Lin, Y. H. & Teng, S. C. Rapid Cdc13 turnover and telomere length homeostasis are controlled by Cdk1-mediated phosphorylation of Cdc13. Nucleic Acids Res. 37, 3602–3611 (2009).
Li, S. et al. Cdk1-dependent phosphorylation of Cdc13 coordinates telomere elongation during cell-cycle progression. Cell 136, 50–61 (2009).
Taggart, A. K., Teng, S. C. & Zakian, V. A. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science 297, 1023–1026 (2002). Demonstrates that Est1, Est2 and Cdc13 are associated with telomeres during telomere replication in the S phase of the cell cycle and suggests a telomerase-activating function for Est1 upon binding to Cdc13.
Tuzon, C. T., Wu, Y., Chan, A. & Zakian, V. A. The Saccharomyces cerevisiae telomerase subunit Est3 binds telomeres in a cell cycle- and Est1-dependent manner and interacts directly with Est1 in vitro. PLoS Genet. 7, e1002060 (2011).
DeZwaan, D. C. & Freeman, B. C. The conserved Est1 protein stimulates telomerase DNA extension activity. Proc. Natl Acad. Sci. USA 106, 17337–17342 (2009).
DeZwaan, D. C. & Freeman, B. C. Is there a telomere-bound 'EST' telomerase holoenzyme? Cell Cycle 9, 1913–1917 (2010).
Lee, J., Mandell, E. K., Tucey, T. M., Morris, D. K. & Lundblad, V. The Est3 protein associates with yeast telomerase through an OB-fold domain. Nature Struct. Mol. Biol. 15, 990–997 (2008).
Yu, E. Y., Wang, F., Lei, M. & Lue, N. F. A proposed OB-fold with a protein-interaction surface in Candida albicans telomerase protein Est3. Nature Struct. Mol. Biol. 15, 985–989 (2008).
Yen, W. F., Chico, L., Lei, M. & Lue, N. F. Telomerase regulatory subunit Est3 in two Candida species physically interacts with the TEN domain of TERT and telomeric DNA. Proc. Natl Acad. Sci. USA 108, 20370–20375 (2011).
Fisher, T. S., Taggart, A. K. & Zakian, V. A. Cell cycle-dependent regulation of yeast telomerase by Ku. Nature Struct. Mol. Biol. 11, 1198–1205 (2004).
Bertuch, A. A. & Lundblad, V. The Ku heterodimer performs separable activities at double-strand breaks and chromosome termini. Mol. Cell. Biol. 23, 8202–8215 (2003).
Pfingsten, J. S. et al. Mutually exclusive binding of telomerase RNA and DNA by Ku alters telomerase recruitment model. Cell 148, 922–932 (2011).
Gallardo, F., Olivier, C., Dandjinou, A. T., Wellinger, R. J. & Chartrand, P. TLC1 RNA nucleo–cytoplasmic trafficking links telomerase biogenesis to its recruitment to telomeres. EMBO J. 27, 748–757 (2008).
Schober, H., Ferreira, H., Kalck, V., Gehlen, L. R. & Gasser, S. M. Yeast telomerase and the SUN domain protein Mps3 anchor telomeres and repress subtelomeric recombination. Genes Dev. 23, 928–938 (2009).
Ferreira, H. C. et al. The PIAS homologue Siz2 regulates perinuclear telomere position and telomerase activity in budding yeast. Nature Cell Biol. 13, 867–874 (2011).
Gottschling, D. E., Aparicio, O. M., Billington, B. L. & Zakian, V. A. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell 63, 751–762 (1990).
Mishra, K. & Shore, D. Yeast Ku protein plays a direct role in telomeric silencing and counteracts inhibition by Rif proteins. Curr. Biol. 9, 1123–1126 (1999).
Lopez, C. R. et al. Ku must load directly onto the chromosome end in order to mediate its telomeric functions. PLoS Genet. 7, e1002233 (2011).
Ribes-Zamora, A., Mihalek, I., Lichtarge, O. & Bertuch, A. A. Distinct faces of the Ku heterodimer mediate DNA repair and telomeric functions. Nature Struct. Mol. Biol. 14, 301–307 (2007).
Stansel, R. M., de Lange, T. & Griffith, J. D. T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. EMBO J. 20, 5532–5540 (2001).
Zaug, A. J., Podell, E. R. & Cech, T. R. Human POT1 disrupts telomeric G-quadruplexes allowing telomerase extension in vitro. Proc. Natl Acad. Sci. USA 102, 10864–10869 (2005).
Pedroso, I. M., Hayward, W. & Fletcher, T. M. The effect of the TRF2 N-terminal and TRFH regions on telomeric G-quadruplex structures. Nucleic Acids Res. 37, 1541–1554 (2009).
Wang, H., Nora, G. J., Ghodke, H. & Opresko, P. L. Single molecule studies of physiologically relevant telomeric tails reveal POT1 mechanism for promoting G-quadruplex unfolding. J. Biol. Chem. 286, 7479–7489 (2011).
Raghuraman, M. K. & Cech, T. R. Effect of monovalent cation-induced telomeric DNA structure on the binding of Oxytricha telomeric protein. Nucleic Acids Res. 18, 4543–4552 (1990).
Yanez, G. H., Khan, S. J., Locovei, A. M., Pedroso, I. M. & Fletcher, T. M. DNA structure-dependent recruitment of telomeric proteins to single-stranded/double-stranded DNA junctions. Biochem. Biophys. Res. Commun. 328, 49–56 (2005).
Schaffitzel, C. et al. In vitro generated antibodies specific for telomeric guanine-quadruplex DNA react with Stylonychia lemnae macronuclei. Proc. Natl Acad. Sci. USA 98, 8572–8577 (2001).
Zahler, A. M., Williamson, J. R., Cech, T. R. & Prescott, D. M. Inhibition of telomerase by G-quartet DNA structures. Nature 350, 718–720 (1991).
Wang, Q. et al. G-quadruplex formation at the 3′ end of telomere DNA inhibits its extension by telomerase, polymerase and unwinding by helicase. Nucleic Acids Res. 39, 6229–6237 (2011).
Acknowledgements
T.R.C. is an investigator at the Howard Hughes Medical Institute. The telomerase research in the author's laboratory is funded in part by the US National Institutes of Health (NIH) grants R01 GM099705 to T.R.C. and K99 CA167644 to J.N.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Thomas R. Cech and Jayakrishnan Nandakumarare inventors on a patent application relating to the identification of a patch on TPP1 that mediates telomerase recruitment.
Related links
Glossary
- Dyskeratosis congenita
-
A rare genetic disease, characterized by abnormal skin pigmentation, nail dystrophy and mucosal leukoplakia, caused by telomere shortening owing to mutations in telomerase core components (telomerase reverse transcriptase (TERT) and a template-containing RNA component (TR)), telomerase accessory factors (such as dyskerin, telomerase Cajal body protein 1 (TCAB1), NHP2 and nucleolar protein 10 (Nop10)), or the shelterin protein TRF1-interacting nuclear protein 2 (TIN2).
- Hoogsteen base-pairing
-
Non-Watson–Crick pairing that involves the N7 atom of a purine and is important in stabilizing triplex and quadruplex nucleic acid structures.
- Cajal bodies
-
Conserved nuclear organelles that are involved in the biogenesis of small nuclear ribonucleoprotein (snRNP) particles and the maturation of the telomerase RNP.
- Sm proteins
-
Proteins that form a ring which binds to a specific U-rich sequence near the 3′ ends of small nuclear RNAs involved in mRNA splicing, allowing nuclear import of the ribonucleoprotein.
- T-motif
-
An amino acid sequence preceding the reverse transcriptase motif that is conserved among telomerase reverse transcriptase (TERT) proteins but not apparent in other reverse transcriptases.
- Photocrosslink
-
The formation of covalent adducts between nucleotides and/or amino acids that are adjacent in a complex using light energy, generally in the ultraviolet range.
- Immunofluorescence
-
Localization of a protein (or other macromolecule) within cells or tissues using a specific antibody that is derivatized with a fluorescent probe (direct immunofluorescence) or using a fluorescently labelled secondary antibody (indirect immunofluorescence).
- Fluorescence in situ hybridization
-
(FISH). Localization of specific nucleic acid sequences in the cell by specific annealing of fluorescently labelled antisense oligonucleotide probes.
- Chromatin immunoprecipitation
-
(ChIP). A technique that allows the isolation of DNA sequences bound to a protein of interest using specific antibodies.
- Non-homologous end-joining
-
(NHEJ). A repair pathway of DNA double-strand breaks by directly ligating the broken ends without the need for a homologous template.
Rights and permissions
About this article
Cite this article
Nandakumar, J., Cech, T. Finding the end: recruitment of telomerase to telomeres. Nat Rev Mol Cell Biol 14, 69–82 (2013). https://doi.org/10.1038/nrm3505
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3505
This article is cited by
-
Nucleotide metabolism regulates human telomere length via telomerase activation
Nature Genetics (2023)
-
Conformational plasticity and allosteric communication networks explain Shelterin protein TPP1 binding to human telomerase
Communications Chemistry (2023)
-
Oxidative Stress, Telomere Length and Telomerase Activity in Spermatogenesis Disorders (Review of Scientific Activity)
Bulletin of Experimental Biology and Medicine (2023)
-
TGF-β controls stromal telomere length through epigenetic modifications
3 Biotech (2022)
-
Telomere length in patients with osteoarthritis: a systematic review and meta-analysis
Aging Clinical and Experimental Research (2022)