Key Points
-
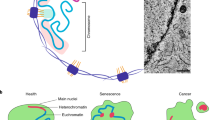
Heterochromatin, euchromatin and the nuclear matrix are often collectively referred to as the nuclear architecture. Changes in nuclear architecture appear to be an evolutionarily conserved hallmark of ageing that may result in increased genomic instability as well as transcriptional deregulation.
-
In yeast, ageing is a direct consequence of increased genomic instability in ribosomal DNA (rDNA). The NAD+-dependent histone deacetylase Sir2 has a crucial role in heterochromatin formation in budding yeast by stabilizing rDNA and thereby extending lifespan.
-
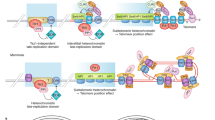
DNA damage and increased rDNA instability trigger the redistribution of Sir2-containing DNA-silencing complexes from heterochromatin to sites of DNA damage. This results in a loss of silencing at functionally important loci — telomeres, rDNA and mating-type loci — and phenotypic changes such as sterility, which together are manifested as yeast ageing.
-
Human premature-ageing syndromes implicate increased genomic instability and alterations in nuclear architecture in normal human ageing. Cells from older humans and cells that have undergone DNA-damage-induced senescence show significant changes in heterochromatin, including loss of perinuclear heterochromatin and the formation of senescence-associated heterochromatin foci (SAHFs).
-
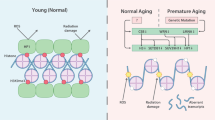
Changes in gene expression are a hallmark of ageing across species and may directly contribute to the ageing process by impairing the ability of a cell to function normally. Changes in nuclear architecture caused by DNA damage may underlie these changes.
-
Like in yeast, mammalian DNA-damage repair requires the recruitment of chromatin-modifying enzymes to sites of DNA damage. We propose that DNA damage triggers an evolutionarily conserved redistribution of chromatin modifiers that aids DNA repair but may result in loss of silencing at other loci, thereby explaining age-related gene-expression changes. We refer to this as the epigenetic balance hypothesis of ageing.
Abstract
Eukaryotes come in many shapes and sizes, yet one thing that they all seem to share is a decline in vitality and health over time — a process known as ageing. If there are conserved causes of ageing, they may be traced back to common biological structures that are inherently difficult to maintain throughout life. One such structure is chromatin, the DNA–protein complex that stabilizes the genome and dictates gene expression. Studies in the budding yeast Saccharomyces cerevisiae have pointed to chromatin reorganization as a main contributor to ageing in that species, which raises the possibility that similar processes underlie ageing in more complex organisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Cheutin, T. et al. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science 299, 721–725 (2003).
Grewal, S. I. & Jia, S. Heterochromatin revisited. Nature Rev. Genet. 8, 35–46 (2007).
Obe, G. et al. Chromosomal aberrations: formation, identification and distribution. Mutat. Res. 504, 17–36 (2002).
Villeponteau, B. The heterochromatin loss model of aging. Exp. Gerontol. 32, 383–394 (1997).
Imai, S. & Kitano, H. Heterochromatin islands and their dynamic reorganization: a hypothesis for three distinctive features of cellular aging. Exp. Gerontol. 33, 555–570 (1998).
Goldstein, S. Replicative senescence: the human fibroblast comes of age. Science 249, 1129–1133 (1990).
Campisi, J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120, 513–522 (2005).
Kennedy, B. K. et al. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell 89, 381–391 (1997).
Sinclair, D. A. & Guarente, L. Extrachromosomal rDNA circles — a cause of aging in yeast. Cell 91, 1033–1042 (1997).
Chakalova, L., Debrand, E., Mitchell, J. A., Osborne, C. S. & Fraser, P. Replication and transcription: shaping the landscape of the genome. Nature Rev. Genet. 6, 669–677 (2005).
Hennekam, R. C. Hutchinson–Gilford progeria syndrome: review of the phenotype. Am. J. Med. Genet. A 140, 2603–2624 (2006).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003). Describes how cellular senescence leads to the formation of facultative heterochromatic foci, which alter the nuclear architecture. Alterations in nuclear architecture change the expression of cell-cycle regulators and can cause cell-cycle arrest.
Scaffidi, P. & Misteli, T. Lamin A-dependent nuclear defects in human aging. Science 312, 1059–1063 (2006). Implicates the main cause of HGPS — a defective lamin A splice variant — in normal human ageing.
Sinclair, D. A., Mills, K. & Guarente, L. Molecular mechanisms of yeast aging. Trends Biochem. Sci. 23, 131–134 (1998).
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000).
Kaeberlein, M., McVey, M. & Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13, 2570–2580 (1999).
Straight, A. F. et al. Net1, a Sir2-associated nucleolar protein required for rDNA silencing and nucleolar integrity. Cell 97, 245–256 (1999).
Shou, W. et al. Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 97, 233–244 (1999).
Visintin, R. et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell 2, 709–718 (1998).
Kennedy, B. K., Austriaco, N. R., Jr, Zhang, J. & Guarente, L. Mutation in the silencing gene SIR4 can delay aging in S. cerevisiae. Cell 80, 485–496 (1995).
Watt, P. M., Louis, E. J., Borts, R. H. & Hickson, I. D. Sgs1: a eukaryotic homolog of E. coli RecQ that interacts with topoisomerase II in vivo and is required for faithful chromosome segregation. Cell 81, 253–260 (1995).
Yu, C. E. et al. Positional cloning of the Werner's syndrome gene. Science 272, 258–262 (1996).
Sinclair, D. A., Mills, K. & Guarente, L. Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science 277, 1313–1316 (1997).
McAinsh, A. D., Scott-Drew, S., Murray, J. A. & Jackson, S. P. DNA damage triggers disruption of telomeric silencing and Mec1p-dependent relocation of Sir3p. Curr. Biol. 9, 963–966 (1999).
Mills, K. D., Sinclair, D. A. & Guarente, L. MEC1-dependent redistribution of the Sir3 silencing protein from telomeres to DNA double-strand breaks. Cell 97, 609–620 (1999).
Lee, S. E., Paques, F., Sylvan, J. & Haber, J. E. Role of yeast SIR genes and mating type in directing DNA double-strand breaks to homologous and non-homologous repair paths. Curr. Biol. 9, 767–770 (1999).
Martin, S. G., Laroche, T., Suka, N., Grunstein, M. & Gasser, S. M. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell 97, 621–633 (1999). References 25 and 27 show that DNA damage triggers the relocalization of the yeast Sir-silencing complex to sites of DNA breaks and causes nuclear changes that are reminiscent of normal ageing in yeast.
Tamburini, B. A. & Tyler, J. K. Localized histone acetylation and deacetylation triggered by the homologous recombination pathway of double-strand DNA repair. Mol. Cell. Biol. 25, 4903–4913 (2005).
McMurray, M. A. & Gottschling, D. E. An age-induced switch to a hyper-recombinational state. Science 301, 1908–1911 (2003).
Eriksson, M. et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature 423, 293–298 (2003).
Scaffidi, P. & Misteli, T. Reversal of the cellular phenotype in the premature aging disease Hutchinson–Gilford progeria syndrome. Nature Med. 11, 440–445 (2005).
Haithcock, E. et al. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 102, 16690–16695 (2005).
Shiloh, Y. Ataxia-telangiectasia: closer to unraveling the mystery. Eur. J. Hum. Genet. 3, 116–138 (1995).
Smilenov, L. B. et al. Influence of ATM function on telomere metabolism. Oncogene 15, 2659–2665 (1997).
Verdun, R. E. & Karlseder, J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell 127, 709–720 (2006).
Greenwell, P. W. et al. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell 82, 823–829 (1995).
Gaubatz, J. W. & Cutler, R. G. Mouse satellite DNA is transcribed in senescent cardiac muscle. J. Biol. Chem. 265, 17753–17758 (1990).
Shen, S., Liu, A., Li, J., Wolubah, C. & Casaccia-Bonnefil, P. Epigenetic memory loss in aging oligodendrocytes in the corpus callosum. Neurobiol. Aging 19 December 2006 (doi:10.1016/j.neurobiolaging.2006.10.026).
Imai, S. et al. Dissociation of Oct-1 from the nuclear peripheral structure induces the cellular aging-associated collagenase gene expression. Mol. Biol. Cell 8, 2407–2419 (1997).
Zhang, R. et al. Formation of macroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev. Cell 8, 19–30 (2005).
Dellaire, G. & Bazett-Jones, D. P. PML nuclear bodies: dynamic sensors of DNA damage and cellular stress. Bioessays 26, 963–977 (2004).
Herbig, U., Ferreira, M., Condel, L., Carey, D. & Sedivy, J. M. Cellular senescence in aging primates. Science 311, 1257 (2006).
Lu, T. et al. Gene regulation and DNA damage in the ageing human brain. Nature 429, 883–891 (2004). Gene-expression profiling reveals DNA-damage-induced global gene repression in the ageing brain.
Lee, C. K., Weindruch, R. & Prolla, T. A. Gene-expression profile of the ageing brain in mice. Nature Genet. 25, 294–297 (2000).
Lee, C. K., Klopp, R. G., Weindruch, R. & Prolla, T. A. Gene expression profile of aging and its retardation by caloric restriction. Science 285, 1390–1393 (1999).
Kayo, T., Allison, D. B., Weindruch, R. & Prolla, T. A. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc. Natl Acad. Sci. USA 98, 5093–5098 (2001).
Fraser, H. B., Khaitovich, P., Plotkin, J. B., Paabo, S. & Eisen, M. B. Aging and gene expression in the primate brain. PLoS Biol. 3, e274 (2005).
Park, S. K. & Prolla, T. A. Gene expression profiling studies of aging in cardiac and skeletal muscles. Cardiovasc. Res. 66, 205–212 (2005).
Bahar, R. et al. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature 441, 1011–1014 (2006). Gene-expression patterns vary between individual cells of aged cardiomyocytes. These changes appear to be stochastic and are caused by DNA damage.
Pletcher, S. D. et al. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr. Biol. 12, 712–723 (2002).
Lin, S. J., Defossez, P. A. & Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289, 2126–2128 (2000).
Rogina, B. & Helfand, S. L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl Acad. Sci. USA 101, 15998–16003 (2004).
Cohen, H. Y. et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305, 390–392 (2004).
Pruitt, K. et al. Inhibition of SIRT1 reactivates silenced cancer genes without loss of promoter DNA hypermethylation. PLoS Genet. 2, e40 (2006).
Bordone, L. et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic b cells. PLoS Biol. 4, e31 (2006).
Picard, F. et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature 429, 771–776 (2004).
Yu, B. P. & Chung, H. Y. Adaptive mechanisms to oxidative stress during aging. Mech. Ageing Dev. 127, 436–443 (2006).
Liu, B. et al. Genomic instability in laminopathy-based premature aging. Nature Med. 11, 780–785 (2005).
Csoka, A. B. et al. Genome-scale expression profiling of Hutchinson–Gilford progeria syndrome reveals widespread transcriptional misregulation leading to mesodermal/mesenchymal defects and accelerated atherosclerosis. Aging Cell 3, 235–243 (2004).
Niedernhofer, L. J. et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444, 1038–1043 (2006). A mouse model for a human nucleotide-excision repair defect shows premature ageing and gene-expression changes similar to those observed during normal ageing.
van Attikum, H. & Gasser, S. M. The histone code at DNA breaks: a guide to repair? Nature Rev. Mol. Cell Biol. 6, 757–765 (2005).
Downs, J. A. et al. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol. Cell 16, 979–990 (2004).
Burma, S., Chen, B. P., Murphy, M., Kurimasa, A. & Chen, D. J. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 276, 42462–42467 (2001).
Ward, I. M. & Chen, J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J. Biol. Chem. 276, 47759–47762 (2001).
Rogakou, E. P., Boon, C., Redon, C. & Bonner, W. M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell Biol. 146, 905–916 (1999).
Sanders, S. L. et al. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell 119, 603–614 (2004).
Giannattasio, M., Lazzaro, F., Plevani, P. & Muzi-Falconi, M. The DNA damage checkpoint response requires histone H2B ubiquitination by Rad6–Bre1 and H3 methylation by Dot1. J. Biol. Chem. 280, 9879–9886 (2005).
Kim, S., Benguria, A., Lai, C. Y. & Jazwinski, S. M. Modulation of life-span by histone deacetylase genes in Saccharomyces cerevisiae. Mol. Biol. Cell 10, 3125–3136 (1999).
Huyen, Y. et al. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature 432, 406–411 (2004). The crystal structure of a methyl-binding DNA repair factor reveals an evolutionarily conserved role for histone methylation in mammalian DNA repair.
Botuyan, M. V. et al. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 127, 1361–1373 (2006).
Malins, D. C. et al. Oxidative changes in the DNA of stroma and epithelium from the female breast: potential implications for breast cancer. Cell Cycle 5, 1629–1632 (2006).
Raghavan, S. C. & Lieber, M. R. DNA structures at chromosomal translocation sites. Bioessays 28, 480–494 (2006).
Welle, S., Brooks, A. I., Delehanty, J. M., Needler, N. & Thornton, C. A. Gene expression profile of aging in human muscle. Physiol. Genomics 14, 149–159 (2003).
Harman, D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 (1956).
Loden, M. & van Steensel, B. Whole-genome views of chromatin structure. Chromosome Res. 13, 289–298 (2005).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Craig, J. M. Heterochromatin — many flavours, common themes. Bioessays 27, 17–28 (2005).
Dernburg, A. F. et al. Perturbation of nuclear architecture by long-distance chromosome interactions. Cell 85, 745–759 (1996).
Kovtun, I. V. et al. OGG1 initiates age-dependent CAG trinucleotide expansion in somatic cells. Nature 447, 447–452 (2007).
Bokov, A., Chaudhuri, A. & Richardson, A. The role of oxidative damage and stress in aging. Mech. Ageing Dev. 125, 811–826 (2004).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006).
Lombard, D. B. et al. DNA repair, genome stability, and aging. Cell 120, 497–512 (2005).
Van Remmen, H. et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genomics 16, 29–37 (2003).
Lund, J. et al. Transcriptional profile of aging in C. elegans. Curr. Biol. 12, 1566–1573 (2002).
Hoppe, G. J. et al. Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol. Cell. Biol. 22, 4167–4180 (2002).
Acknowledgements
We thank B. North for critical reading of the manuscript. The Sinclair laboratory is supported by National Institutes of Health grants and the Paul F. Glenn Laboratories for the Biological Mechanisms of Aging. P.O. is supported by the National Space Biomedical Research Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
David A. Sinclair is a co-founder of and consultant to Sirtris Pharmaceuticals, Inc. (USA), a company that aims to treat diseases by modulating sirtuins. He sits on the board of directors and scientific advisory board, and owns less than 1% equity.
Related links
Glossary
- Senescence
-
A nearly irreversible stage of permanent G1 cell-cycle arrest, which is linked to morphological changes, metabolic changes and changes in gene expression. The induction of senescence depends on p53 and cell-cycle inhibitors such as p21 and p16.
- Mating-type locus
-
The mating of yeast only occurs between haploids, which can be either mating type a or mating type α. The mating type is determined by a single locus (MAT). Gene conversion between MAT and the silent mating-type loci HML and HMR allows haploid yeast to switch to the active mating type as often as every cell cycle.
- Telomeres
-
Regions of highly repetitive DNA at the ends of linear chromosomes. Telomeres function as caps to protect the DNA ends from degradation or fusion with other chromosomes, and as facilitators of DNA replication at the ends of chromosomes by recruiting the reverse transcriptase telomerase.
- Progeroid disease
-
A genetic disorder in which various tissues, organs or systems of the human body appear to age prematurely. These diseases are often called segmental progeroid diseases because they do not fully recapitulate normal ageing. A common feature of such diseases is genomic instability.
- RecQ DNA helicase
-
One of a family of DNA helicases that help to stabilize replication forks and remove DNA recombination intermediates, thereby maintaining genome integrity. In humans, there are five family members; mutations in three of these helicases are associated with a predisposition to cancer and premature ageing.
- Position-effect variegation
-
The variation in gene expression that can occur between genetically identical cells when a gene is juxtaposed to a region of contracting and expanding heterochromatin.
- Transcription-coupled repair
-
A DNA-repair mechanism that operates in tandem with transcription and involves members of the XP gene family. Failure of the transcription-coupled repair mechanism results in Cockayne syndrome, an extreme form of accelerated ageing that is fatal early in life.
- Base-excision repair
-
(BER). A DNA-repair pathway that corrects single mutated bases. The two main enzymes used in BER are DNA glycosylases and apurinic or apyrimidinic (AP) endonucleases. The DNA glycosylase hydrolyses the glycosidic bond to create an AP site, which is then recognized and excised by the AP endonuclease, allowing DNA polymerases to replace the missing base.
- Xeroderma pigmentosa
-
A genetic DNA-repair disorder in which the ability of the body to remove damage caused by ultraviolet light is impaired, leading to multiple basaliomas and other skin malignancies at a young age.
Rights and permissions
About this article
Cite this article
Oberdoerffer, P., Sinclair, D. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol 8, 692–702 (2007). https://doi.org/10.1038/nrm2238
Issue Date:
DOI: https://doi.org/10.1038/nrm2238
This article is cited by
-
Current status and challenges of breast cancer prevention~DNA methylation would lead to groundbreaking progress in breast cancer prevention~
Genes and Environment (2023)
-
Mechanisms, pathways and strategies for rejuvenation through epigenetic reprogramming
Nature Aging (2023)
-
Estimating breast tissue-specific DNA methylation age using next-generation sequencing data
Clinical Epigenetics (2020)
-
Stabilizing heterochromatin by DGCR8 alleviates senescence and osteoarthritis
Nature Communications (2019)
-
Giant cells and osteoclasts present in bone grafted with nacre differ by nuclear cytometry evaluated by texture analysis
Journal of Materials Science: Materials in Medicine (2019)