Key Points
-
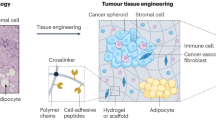
Over the past two decades, the field of tissue engineering has focused primarily on the creation of tissues for patients. The emphasis of the field is now shifting to include the creation of complex in vitro tissue models that help to explain disease processes (for example, breast cancer) and serve as tools to assess the safety and efficacy of therapies (for example, screens of liver toxicity).
-
The 3D extracellular-matrix (ECM) environment in vivo provides both chemical and physical cues to regulate cell behaviour, serving not only as a structural support, but as a depot of many effector molecules. New synthetic matrices that include well-defined adhesion, growth factor and degradation moieties are being developed to mimic these cues, thereby allowing the quantitative analysis of cell migration, differentiation, survival and growth.
-
Gradients of nutrients and effector molecules are present in 3D cultures. The magnitude of gradients for vital molecules such as oxygen can be predicted for a given experimental arrangement, but data are just emerging for the rates of production and consumption of growth factors, cytokines and other effector molecules.
-
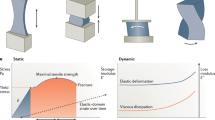
All tissues are subjected to mechanical forces that arise from interstitial flow and tissue movement. These mechanical forces can redistribute effector molecules that are secreted by cells, resulting in the coupling of chemical and mechanical signalling.
-
Microfabrication methods that have been adapted from the microelectronics industry and applied to miniaturize biochemical analyses are now being applied to create complex 3D tissue structures for in vitro studies, and are being combined with microfluidic pumps that can provide microscale fluid flows through tissues for long-term culture.
-
Experimental systems must be developed hand-in-hand with mathematical models that take into account the integration of numerous cues that influence downstream signals and, ultimately, cell responses.
Abstract
The emergence of tissue engineering raises new possibilities for the study of complex physiological and pathophysiological processes in vitro. Many tools are now available to create 3D tissue models in vitro, but the blueprints for what to make have been slower to arrive. We discuss here some of the 'design principles' for recreating the interwoven set of biochemical and mechanical cues in the cellular microenvironment, and the methods for implementing them. We emphasize applications that involve epithelial tissues for which 3D models could explain mechanisms of disease or aid in drug development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lysaght, M. J. & Hazlehurst, A. L. Tissue engineering: the end of the beginning. Tissue Eng. 10, 309?320 (2004).
Griffith, L. G. & Naughton, G. Tissue engineering ?current challenges and expanding opportunities. Science 295, 1009?1014 (2002).
Suuronen, E. J., Sheardown, H., Newman, K. D., McLaughlin, C. R. & Griffith, M. Building in vitro models of organs. Int. Rev. Cytol. 244, 137?173 (2005).
Sivaraman, A. et al. A microscale in vitro physiological model of the liver: predictive screens for drug metabolism and enzyme induction. Curr. Drug Metab. 6, 569?592 (2005).
Kuperwasser, C. et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc. Natl Acad. Sci. USA 101, 4966?4971 (2004).
Katoh, M. et al. Expression of human phase II enzymes in chimeric mice with humanized liver. Drug Metab. Dispos. 33, 1333?1340 (2005).
Rangarajan, A., Hong, S. J., Gifford, A. & Weinberg, R. A. Species- and cell type-specific requirements for cellular transformation. Cancer Cell 6, 171?183 (2004).
Watt, F. M. Selective migration of terminally differentiating cells from the basal layer of cultured human epidermis. J. Cell Biol. 98, 16?21 (1984).
Louekari, K. Status and prospects of in vitro tests in risk assessment. Altern. Lab. Anim. 32, 431?435 (2004).
Knight, B. et al. Visualizing muscle cell migration in situ. Curr. Biol. 10, 576?585 (2000).
Roskelley, C. D., Desprez, P. Y. & Bissell, M. J. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl Acad. Sci. USA 91, 12378?12382 (1994). Seminal work that links mammary phenotype to 3D-culture conditions.
Bissell, M. J., Rizki, A. & Mian, I. S. Tissue architecture: the ultimate regulator of breast epithelial function. Curr. Opin. Cell Biol. 15, 753?762 (2003).
Debnath, J. & Brugge, J. S. Modelling glandular epithelial cancers in three-dimensional cultures. Nature Rev. Cancer 5, 675?688 (2005).
Paszek, M. J. & Weaver, V. M. The tension mounts: mechanics meets morphogenesis and malignancy. J. Mammary Gland Biol. Neoplasia 9, 325?342 (2004).
Wozniak, M. A., Desai, R., Solski, P. A., Der, C. J. & Keely, P. J. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J. Cell Biol. 163, 583?595 (2003).
Zegers, M. M., O'Brien, L. E., Yu, W., Datta, A. & Mostov, K. E. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 13, 169?176 (2003).
Grinnell, F., Ho, C. H., Lin, Y.-C. & Skuta, G. Differences in the regulation of fibroblast contraction of floating versus stressed collagen matrices. J. Biol. Chem. 274, 918?923 (1999).
Zaman, M. H., Kamm, R. D., Matsudaira, P. & Lauffenburger, D. A. Computational model for cell migration in three-dimensional matrices. Biophys. J. 89, 1389?1397 (2005).
Lutolf, M. P. et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl Acad. Sci. USA 100, 5413?5418 (2003). Pioneering demonstration of how synthetic extracellular matrices can be tuned to control many facets of cell behaviour in tissue remodelling, using approaches that are accessible to the general cell-biology laboratory.
Lo, C. M., Wang, H. B., Dembo, M. & Wang, Y. L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144?152 (2000).
Peyton, S. R. & Putnam, A. J. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J. Cell Physiol. 204, 198?209 (2005).
Sieminski, A. L., Hebbel, R. P. & Gooch, K. J. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp. Cell Res. 297, 574?584 (2004).
Muschler, J. et al. A role for dystroglycan in epithelial polarization: loss of function in breast tumor cells. Cancer Res. 62, 7102?7109 (2002).
Paszek, M. J. et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241?254 (2005). Elegant demonstration of a link between matrix mechanics and phenotype in normal and malignant mammary tissue, using a comprehensive range of innovative methods to systematically vary matrix properties in vitro to match measured in vivo properties, as well as to quantify cell responses.
Wozniak, M. A. & Keely, P. J. Use of three-dimensional collagen gels to study mechanotransduction in T47D breast epithelial cells. Biol. Proced. Online 7, 144?161 (2005).
Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C. & Brown, R. A. Myofibroblasts and mechanoregulation of connective tissue remodelling. Nature Rev. Mol. Cell Biol. 3, 349?363 (2002).
Shreiber, D. I., Barocas, V. H. & Tranquillo, R. T. Temporal variations in cell migration and traction during fibroblast-mediated gel compaction. Biophys. J. 84, 4102?4114 (2003).
Tsai, K. K., Chuang, E. Y., Little, J. B. & Yuan, Z. M. Cellular mechanisms for low-dose ionizing radiation-induced perturbation of the breast tissue microenvironment. Cancer Res. 65, 6734?6744 (2005).
Paralkar, V. M., Vukicevic, S. & Reddi, A. H. Transforming growth factor β type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev. Biol. 143, 303?308 (1991).
Ruhrberg, C. et al. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 16, 2684?2698 (2002). This paper showed that heparin-binding VEGF isoforms were necessary for endothelial branching, using transgenic mice that expressed only either matrix-interacting or non-interacting VEGF isoforms.
Tschumperlin, D. et al. Mechanotransduction through growth factor shedding into the extracellular space. Nature 429, 83?86 (2004).
Swartz, M. A. Signaling in morphogenesis: transport cues in morphogenesis. Curr. Opin. Biotech. 14, 547?550 (2003).
Lutolf, M. P. & Hubbell, J. A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnol. 23, 47?55 (2005).
Wang, Y. L. & Pelham, R. J. Jr . Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Methods Enzymol. 298, 489?496 (1998).
Reinhart-King, C. A., Dembo, M. & Hammer, D. A. The dynamics and mechanics of endothelial cell spreading. Biophys. J. 89, 676?689 (2005).
Semler, E. J., Lancin, P. A., Dasgupta, A. & Moghe, P. V. Engineering hepatocellular morphogenesis and function via ligand-presenting hydrogels with graded mechanical compliance. Biotechnol. Bioeng. 89, 296?307 (2005).
Kong, H. J., Polte, T. R., Alsberg, E. & Mooney, D. J. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc. Natl Acad. Sci. USA 102, 4300?4305 (2005).
Semino, C. E., Merok, J. R., Crane, G. G., Panagiotakos, G. & Zhang, S. Functional differentiation of hepatocyte-like spheroid structures from putative liver progenitor cells in three-dimensional peptide scaffolds. Differentiation 71, 262?270 (2003).
Kisiday, J. et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc. Natl Acad. Sci. USA 99, 9996?10001 (2002).
Raeber, G. P., Lutolf, M. P. & Hubbell, J. A. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys. J. 89, 1374?1388 (2005).
Maheshwari, G., Brown, G., Lauffenburger, D. A., Wells, A. & Griffith, L. G. Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 113, 1677?1686 (2000).
Coussen, F., Choquet, D., Sheetz, M. P. & Erickson, H. P. Trimers of the fibronectin cell adhesion domain localize to actin filament bundles and undergo rearward translocation. J. Cell Sci. 115, 2581?2590 (2002).
Orend, G. Potential oncogenic action of tenascin-C in tumorigenesis. Int. J. Biochem. Cell Biol. 37, 1066?1083 (2005).
Ehrbar, M. et al. Cell-demanded liberation of VEGF121 from fibrin implants induces local and controlled blood vessel growth. Circ. Res. 94, 1124?1132 (2004). Using protein engineering, this study showed that matrix-binding forms of growth factors led to different signalling patterns, compared with free or unbound growth factors.
Helm, C. E., Fleury, M. E., Zisch, A. H., Boschetti, F. & Swartz, M. A. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc. Natl Acad. Sci. USA 44, 15779?15784 (2005).
Gobin, A. S. & West, J. L. Effects of epidermal growth factor on fibroblast migration through biomimetic hydrogels. Biotechnol. Prog. 19, 1781?1785 (2003).
Rosner, B. I., Hang, T. & Tranquillo, R. T. Schwann cell behavior in three-dimensional collagen gels: evidence for differential mechano-transduction and the influence of TGF-β1 in morphological polarization and differentiation. Exp. Neurol. 195, 81?91 (2005).
Kellner, K. et al. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol. Bioeng. 80, 73?83 (2002).
Glicklis, R., Merchuk, J. C. & Cohen, S. Modeling mass transfer in hepatocyte spheroids via cell viability, spheroid size, and hepatocellular functions. Biotechnol. Bioeng. 86, 672?680 (2004).
Gebhardt, R. et al. New hepatocyte in vitro systems for drug metabolism: metabolic capacity and recommendations for application in basic research and drug development, standard operation procedures. Drug Metab. Rev. 35, 145?213 (2003).
Martin, Y. & Vermette, P. Bioreactors for tissue mass culture: design, characterization, and recent advances. Biomaterials 26, 7481?7503 (2005).
Guppy, M., Leedman, P., Zu, X. & Russell, V. Contribution by different fuels and metabolic pathways to the total ATP turnover of proliferating MCF-7 breast cancer cells. Biochem. J. 364, 309?315 (2002).
Hermitte, F., Brunet de la Grange, P., Belloc, F., Praloran, V. & Ivanovic, Z. Very low O2 concentration (0.1%) favors G0 return of dividing CD34+ cells. Stem Cells 24, 65?73 (2006).
Ezashi, T., Das, P. & Roberts, R. M. Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl Acad. Sci. USA 102, 4783?4788 (2005).
Wang, D. W., Fermor, B., Gimble, J. M., Awad, H. A. & Guilak, F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J. Cell Physiol. 204, 184?191 (2005).
Zhao, F. et al. Effects of oxygen transport on 3-D human mesenchymal stem cell metabolic activity in perfusion and static cultures: experiments and mathematical model. Biotechnol. Prog. 21, 1269?1280 (2005).
Chow, D. C., Wenning, L. A., Miller, W. M. & Papoutsakis, E. T. Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys. J. 81, 685?696 (2001).
Radisky, D. C. et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436, 123?127 (2005).
Wojciak-Stothard, B., Tsang, L. Y. & Haworth, S. G. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 288, L749?L760 (2005).
Morin, J. P., Preterre, D., Keravec, V. & Thuillez, C. Rotating wall vessel as a new in vitro shear stress generation system: application to rat coronary endothelial cell cultures. Cell Biol. Toxicol. 19, 227?242 (2003).
MacDonald, J. M., Wolfe, S. P., Roy-Chowdhury, I., Kubota, H. & Reid, L. M. Effect of flow configuration and membrane characteristics on membrane fouling in a novel multicoaxial hollow-fiber bioartificial liver. Ann. NY Acad. Sci. 944, 334?343 (2001).
Zeilinger, K. et al. Time course of primary liver cell reorganization in three-dimensional high-density bioreactors for extracorporeal liver support: an immunohistochemical and ultrastructural study. Tissue Eng. 10, 1113?1124 (2004).
Reddy, C. C., Niyogi, S. K., Wells, A., Wiley, H. S. & Lauffenburger, D. A. Engineering EGF for enhanced mitogenic potency. Nature Biotechnol. 14, 1696?1699 (1996).
Janowska-Wieczorek, A., Majka, M., Ratajczak, J. & Ratajczak, M. Z. Autocrine/paracrine mechanisms in human hematopoiesis. Stem Cells 19, 99?107 (2001).
Prabhu, S. D. Cytokine-induced modulation of cardiac function. Circ. Res. 95, 1140?1153 (2004).
Singh, A. B. & Harris, R. C. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell. Signal. 17, 1183?1193 (2005).
Janes, K. A. et al. A systems model of signaling identifies a molecular basis set for cytokine-induced apoptosis. Science 310, 1646?1653 (2005).
DeWitt, A. et al. Affinity regulates spatial range of EGF receptor autocrine ligand binding. Dev. Biol. 250, 305?316 (2002). Determined quantitative properties that govern tissue distribution of secreted growth factors.
Wiley, H. S., Shvartsman, S. Y. & Lauffenburger, D. A. Computational modeling of the EGF-receptor system: a paradigm for systems biology. Trends Cell Biol. 13, 43?50 (2003).
Cartmell, S. H., Porter, B. D., Garcia, A. J. & Guldberg, R. E. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 9, 1197?1203 (2003).
Chary, S. R. & Jain, R. K. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc. Natl Acad. Sci. USA 86, 5385?5389 (1989).
Dafni, H., Israely, T., Bhujwalla, Z. M., Benjamin, L. E. & Neeman, M. Overexpression of vascular endothelial growth factor 165 drives peritumor interstitial convection and induces lymphatic drain: magnetic resonance imaging, confocal microscopy, and histological tracking of triple-labeled albumin. Cancer Res. 62, 6731?6739 (2002).
Gurdon, J. B. & Bourillot, P. Y. Morphogen gradient interpretation. Nature 413, 797?803 (2001).
Quinn, T. M., Grodzinsky, A. J., Buschmann, M. D., Kim, Y. J. & Hunziker, E. B. Mechanical compression alters proteoglycan deposition and matrix deformation around individual cells in cartilage explants. J. Cell Sci. 111, 573?583 (1998).
Garcia, A. M., Lark, M. W., Trippel, S. B. & Grodzinsky, A. J. Transport of tissue inhibitor of metalloproteinases-1 through cartilage: Contributions of fluid flow and electrical migration. J. Orthop. Res. 16, 734?742 (1998).
Semino, C. E., Kamm, R. D. & Lauffenburger, D. A. Autocrine EGF receptor activation mediates endothelial cell migration and vascular morphogenesis induced by VEGF under interstitial flow. Exp. Cell Res. 312, 289?298 (2006).
Swartz, M. A., Tschumperlin, D. J., Kamm, R. D. & Drazen, J. M. Mechanical stress is communicated between cell types to elicit matrix remodeling. Proc. Natl Acad. Sci. USA 98, 6180?6185 (2001).
Choe, M. M., Sporn, P. H. S. & Swartz, M. A. An in vitro airway wall model of remodeling. Am. J. Physiol. Lung Cell Physiol. 285, L427?L433 (2003).
Popel, A. S. & Johnson, P. C. Microcirculation and microrheology. Ann. Rev. Fluid Mech. 37, 43?69 (2005).
Schmid-Schonbein, G. W. Biomechanics of microcirculatory blood perfusion. Annu. Rev. Biomed. Eng. 1, 73?102 (1999).
Boardman, K. C. & Swartz, M. A. Interstitial flow as a guide for lymphangiogenesis. Circ. Res. 92, 801?808 (2003).
Ng, C. P., Helm, C. L. & Swartz, M. A. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvasc. Res. 68, 258?264 (2004).
LeCouter, J. et al. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science 299, 890?893 (2003).
Shin, V., Zebboudj, A. F. & Bostrom, K. Endothelial cells modulate osteogenesis in calcifying vascular cells. J. Vasc. Res. 41, 193?201 (2004).
Matsumoto, K., Yoshitomi, H., Roussant, J. & Zaret, K. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294, 559?563 (2001).
Cao, Y. Emerging mechanisms of tumour lymphangiogenesis and lymphatic metastasis. Nature Rev. Cancer 5, 735?743 (2005).
Swartz, M. A. & Skobe, M. Lymphatic function, lymphangiogenesis, and cancer metastasis. Microsc. Res. Tech. 55, 92?99 (2001).
Saharinen, P., Tammela, T., Karkkainen, M. J. & Alitalo, K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 25, 387?395 (2004).
Van Trappen, P. O. & Pepper, M. S. Lymphatic dissemination of tumour cells and the formation of micrometastases. Lancet Oncol. 3, 44?52 (2002).
Balkwill, F. Cancer and the chemokine network. Nature Rev. Cancer 4, 540?550 (2004).
Mougel, L. et al. Three-dimensional culture and multidrug resistance: effects on immune reactivity of MCF-7 cells by monocytes. Anticancer Res. 24, 935?941 (2004).
Elgert, K. D., Alleva, D. G. & Mullins, D. W. Tumor-induced immune dysfunction: The macrophage connection. J. Leukoc. Biol. 64, 275?290 (1998).
Wiley, H. E., Gonzalez, E. B., Maki, W., Wu, M. T. & Hwang, S. T. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J. Natl Cancer Inst. 93, 1638?1643 (2001).
Patel, D. D. et al. Chemokines have diverse abilities to form solid phase gradients. Clin. Immunol. 99, 43?52 (2001).
Goswami, S. et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 65, 5278?5283 (2005). Compelling evidence of a cell-generated chemotactic gradient that operates in 3D on a heterotypic cell type.
Muschler, G. F., Nakamoto, C. & Griffith, L. G. Engineering principles of clinical cell-based tissue engineering. J. Bone Joint Surg. Am. 86-A, 1541?1558 (2004).
Stern, R., McPherson, M. & Longaker, M. T. Histologic study of artificial skin used in the treatment of full-thickness thermal injury. J. Burn Care Rehabil. 11, 7?13 (1990).
Mansbridge, J., Liu, K., Patch, R., Symons, K. & Pinney, E. Three-dimensional fibroblast culture implant for the treatment of diabetic foot ulcers: metabolic activity and therapeutic range. Tissue Eng. 4, 403?414 (1998).
Yannas, I. V. Synthesis of tissues and organs. ChemBioChem 5, 26?39 (2004).
Stock, U. A. & Vacanti, J. P. Tissue engineering: current state and prospects. Annu. Rev. Med. 52, 443?451 (2001).
Yannas, I. V., Lee, E., Orgill, D. P., Skrabut, E. M. & Murphy, G. F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl Acad. Sci. USA 86, 933?937 (1989). Pioneering demonstration of design principles that are applied to the development of synthetic scaffolds for tissue regeneration.
Jakab, K., Neagu, A., Mironov, V., Markwald, R. R. & Forgacs, G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc. Natl Acad. Sci. USA 101, 2864?2869 (2004).
Tremblay, P. L., Hudon, V., Berthod, F., Germain, L. & Auger, F. A. Inosculation of tissue-engineered capillaries with the host's vasculature in a reconstructed skin transplanted on mice. Am. J. Transplant. 5, 1002?1010 (2005).
Levenberg, S. et al. Engineering vascularized skeletal muscle tissue. Nature Biotechnol. 23, 879?884 (2005).
Tlsty, T. D. & Hein, P. W. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr. Opin. Genet. Dev. 11, 54?59 (2001).
Jasmund, I. & Bader, A. Bioreactor developments for tissue engineering applications by the example of the bioartificial liver. Adv. Biochem. Eng. Biotechnol. 74, 99?109 (2002).
Poznansky, M. C. et al. Efficient generation of human T cells from a tissue-engineered thymic organoid. Nature Biotechnol. 18, 729?734 (2000).
Stachowiak, A. N., Bershteyn, A. & Irvine, D. J. Bioactive hydrogels with an ordered cellular structure combine interconnected macroporosity and robust mechanical properties. Adv. Mater. 17, 399?403 (2005). Demonstration of an innovative approach in creating synthetic scaffolds for the recreation of complex tissue behaviours in vitro.
Whitesides, G. M., Ostuni, E., Takayama, S., Jiang, X. & Ingber, D. E. Soft lithography in biology and biochemistry. Annu. Rev. Biomed. Eng. 3, 335?373 (2001).
Klebe, R. J. Cytoscribing: a method for micropositioning cells and the construction of two- and three-dimensional synthetic tissues. Exp. Cell Res. 179, 362?373 (1988).
Tsuda, Y. et al. The use of patterned dual thermoresponsive surfaces for the collective recovery as co-cultured cell sheets. Biomaterials 26, 1885?1893 (2005).
Andersson, H. & van den Berg, A. Microfabrication and microfluidics for tissue engineering: state of the art and future opportunities. Lab Chip 4, 98?103 (2004).
Tan, W. & Desai, T. A. Microscale multilayer cocultures for biomimetic blood vessels. J. Biomed. Mater. Res. A 72, 146?160 (2005).
Shin, M. et al. Endothelialized networks with a vascular geometry in microfabricated poly(dimethyl siloxane). Biomed. Microdevices 6, 269?278 (2004).
Weibel, D. B., Garstecki, P. & Whitesides, G. M. Combining microscience and neurobiology. Curr. Opin. Neurobiol. 15, 560?567 (2005).
Hansen, C. & Quake, S. R. Microfluidics in structural biology: smaller, faster em leader better. Curr. Opin. Struct. Biol. 13, 538?544 (2003).
Lin, F. et al. Neutrophil migration in opposing chemoattractant gradients using microfluidic chemotaxis devices. Ann. Biomed. Eng. 33, 475?482 (2005).
Lu, H. et al. Microfluidic shear devices for quantitative analysis of cell adhesion. Anal. Chem. 76, 5257?5264 (2004).
Song, J. W. et al. Computer-controlled microcirculatory support system for endothelial cell culture and shearing. Anal. Chem. 77, 3993?3999 (2005).
Sin, A. et al. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnol. Prog. 20, 338?345 (2004). Shows the principles of multi-compartment tissue models 'on a chip', incorporating many principles of microfabrication and microfluidics.
Yaakov, N., Schwartz, R. E., Hu, W.-S., Verfaillie, C. & Odde, D. J. Endothelium-mediated hepatocyte recruitment in the establishment of liver-like tissue in vitro. Tissue Eng. (in the press).
DeLeve, L. D., Wang, X., Hu, L., McCuskey, M. K. & McCuskey, R. S. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G757?G763 (2004).
Powers, M. J. et al. Functional behavior of primary rat liver cells in a three-dimensional perfused microarray bioreactor. Tissue Eng. 8, 499?513 (2002).
Powers, M. J. et al. A microfabricated array bioreactor for perfused 3D liver culture. Biotechnol. Bioeng. 78, 257?269 (2002).
Lin, C. Y., Kikuchi, N. & Hollister, S. J. A novel method for biomaterial scaffold internal architecture design to match bone elastic properties with desired porosity. J. Biomech. 37, 623?636 (2004).
Yeong, W. Y., Chua, C. K., Leong, K. F. & Chandrasekaran, M. Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol. 22, 643?652 (2004).
Sherwood, J. K. et al. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials 23, 4739?4751 (2002).
Bornstein, P. & Sage, E. H. Matricellular proteins: extracellular modulators of cell function. Curr. Opin. Cell Biol. 14, 608?616 (2002).
Sigal, S. H., Brill, S., Fiorino, A. S. & Reid, L. M. The liver as a stem cell and lineage system. Am. J. Physiol. 263, G139?G148 (1992).
Acknowledgements
We thank A. Hwa, A. Wells, D. Stolz, S. Watkins, C. Yates, G. Papworth and P.T. So for the use of unpublished photos. We thank D. Lauffenburger, F. Gertler and V. Weaver for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Related links
Glossary
- Feature
-
An architectural or compositional component of a scaffold that delineates a distinct, defined region. For example, features in a scaffold with a honeycomb architecture would include: the walls, the hexagonal channels and the overall shape.
- Acinar
-
Pertaining to a sac-like tissue structure in the shape of an acinus, which is a polarized epithelial layer surrounding a small lumen that contains secretions (such as milk) from epithelial cells.
- Proteoglycans
-
Extracellular matrix components that consist of a protein core and glycosamino side chains. They are huge molecules (>100 MDa) with a high fixed-charge density and are crucial to maintaining the fluid balance and storing growth factors, cytokines and other morphogens in the matrix.
- Matrigel
-
Commercially available extract of the basement membrane-like ECM that is secreted by the murine Engelbreth-Holm-Swarm (EHS) tumour and that is rich in laminin, type IV collagen, heparan sulphate proteoglycans and growth factors. It supports the in vitro formation of tubes from endothelial cells, as well as the in vitro differentiation of many epithelial cell types.
- Convection
-
Transport by fluid flow (as opposed to diffusion); can refer to the transport of fluid or of solute that is dissolved in the fluid and carried by the flow.
- Autocrine loop
-
Mode of growth-factor signalling in which a cell that expresses a particular growth-factor receptor also synthesizes and releases the corresponding ligand, by which the receptor is activated.
- Interstitial flow
-
Flow through or within the 3D extracellular matrix (as opposed to across a surface or within a vessel).
- Starling force
-
A force that drives fluid movement, including gradients or differences in hydrostatic pressure (which drives fluid flow from higher to lower pressures) and osmotic pressure (which drives fluid flow from less concentrated to more concentrated areas).
- Fluid shear stress
-
Mechanical stress on a surface (for example, of a cell or ECM fibre) caused by fluid flow across that surface.
- Angiogenesis
-
The growth of new blood vessels by sprouting from existing vessels in a process that involves endothelial-cell migration and proliferation.
- Glycosaminoglycans
-
Polysaccharide chains of ECM proteoglycans, comprising disaccharide-repeat units with one amino sugar and one negatively charged (carboxylated or sulphated) sugar.
- Resolution
-
The smallest dimensions over which the placement or size of a feature can be controlled during the fabrication of a device or scaffold. There are three measures of resolution: positive feature size (the minimum width of a wall that can be created); negative feature size (the minimum possible width of a channel or hole) and feature placement (how reproducible the spacing is between features).
- Capillary bed
-
A region of tissue that contains a local network of blood microvessels, where the intimate exchange of fluid and molecular components between blood and tissues occurs.
- Sinusoidal capillary
-
A discontinuous capillary that consists of endothelial cells with unusually wide gaps between them, and (partially) lacking a basement membrane. Sinusoidal capillaries can be found in liver, spleen and bone marrow.
Rights and permissions
About this article
Cite this article
Griffith, L., Swartz, M. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7, 211–224 (2006). https://doi.org/10.1038/nrm1858
Issue Date:
DOI: https://doi.org/10.1038/nrm1858
This article is cited by
-
Next-Gen Dual Transcriptomics for Adult Extrapulmonary Tuberculosis Biomarkers and Host–Pathogen Interplay in Human Cells: A Strategic Review
Indian Journal of Microbiology (2024)
-
Three Dimensional Bioprinting for Hepatic Tissue Engineering: From In Vitro Models to Clinical Applications
Tissue Engineering and Regenerative Medicine (2024)
-
Bladder cancer: therapeutic challenges and role of 3D cell culture systems in the screening of novel cancer therapeutics
Cancer Cell International (2023)
-
Evaluating the antioxidant potential of resveratrol-gold nanoparticles in preventing oxidative stress in endothelium on a chip
Scientific Reports (2023)
-
Inflammasomes as regulators of mechano-immunity
EMBO Reports (2023)