Key Points
-
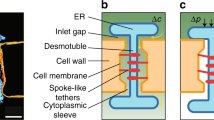
Plasmodesmata are plant-unique intercellular communication channels that acquired the capacity to dilate significantly to allow the trafficking of proteins and RNA within a symplasmic domain. This property is thought to contribute to the supracellular nature of plants.
-
A subset of proteins that regulate the signalling processes beyond the cells in which they are synthesized can traffic through plasmodesmata to function as non-cell-autonomous proteins (NCAPs).
-
One mode of trafficking through plasmodesmata might be controlled by a gate open/gate closed (GO/GC) pathway that leads to the formation of symplasmic domains in which certain unbound molecules (up to ∼40 kDa) can diffuse between neighbouring cells. A second, selective, mode of trafficking through plasmodesmata might involve a specific interaction between each NCAP and the plasmodesmal machinery.
-
Intercellular trafficking of transcription factors that regulate developmental patterning and/or cell-fate determination underscores the important role carried out by the NCAP pathway.
-
RNA can function both as a local and a long-distance information macromolecule by trafficking between cells through plasmodesmata and between organs through the vascular conduit that is provided by phloem. Specific mRNA molecules that can move through phloem might enter various meristematic tissues to redirect developmental events.
-
Phloem translocation has important roles in transmitting RNA-interference signals and systemic signals in response to wounds and pathogen attacks, and in coordinating plant nitrogen metabolism and symbiosis. The formation of a new symplasmic domain between the phloem and a nodule initial illustrates the operation of the integrated signalling system that is provided by plasmodesmata and phloem.
Abstract
The evolution of intercellular communication had an important role in the increasing complexity of both multicellular and supracellular organisms. Plasmodesmata, the intercellular organelles of the plant kingdom, establish an effective pathway for local and long-distance signalling. In higher plants, this pathway involves the trafficking of proteins and various forms of RNA that function non-cell-autonomously to affect developmental programmes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Taga, M. E. & Bassler, B. L. Chemical communication among bacteria. Proc. Natl Acad. Sci. USA 100, 14549–14554 (2003).
Fahrnkrog, B. & Aebi, U. The nuclear pore complex: nucleocytoplasmic transport and beyond. Nature Rev. Mol. Cell. Biol. 4, 757–766 (2003).
Nicholson, B. J. Gap junctions — from cell to molecule. J. Cell Sci. 116, 4479–4481 (2003).
Lucas, W. J., Ding, B. & van der Schoot, C. Plasmodesmata and the supracellular nature of plants. New Phytol. 125, 435–476 (1993).
Franceschi, V. R., Ding, B. & Lucas, W. J. Mechanism of plasmodesmata formation in characean algae in relation to evolution of intercellular communication in higher plants. Planta 192, 347–358 (1994).
Cook, M. E., Graham, L. E., Botha, C. E. J. & Lavin, C. A. Comparative ultrastructure of plasmodesmata of Chara and selected bryophytes: toward an elucidation of the evolutionary origin of plant plasmodesmata. Am. J. Bot. 84, 1169–1178 (1997).
Fujiwara, T., Giesman-Cookmeyer, D., Ding, B., Lommel, S. A. & Lucas, W. J. Cell-to-cell trafficking of macromolecules through plasmodesmata potentiated by the red clover necrotic mosaic virus movement protein. Plant Cell 5, 1783–1794 (1993).
Noueiry, A. O., Lucas, W. J. & Gilbertson, R. L. Two proteins of a plant DNA virus coordinate nuclear and plasmodesmal transport. Cell 76, 925–932 (1994).
Lee, J. -Y., Yoo, B. -C. & Lucas, W. J. Parallels between nuclear-pore and plasmodesmal trafficking of information molecules. Planta 210, 177–187 (2000).
Zambryski, P. & Crawford, K. Plasmodesmata: gatekeepers for cell-to-cell transport of developmental signals in plants. Annu. Rev. Cell Dev. Biol. 16, 393–421 (2000).
Heinlein, M. Plasmodesmata: dynamic regulation and role in macromolecular cell-to-cell signaling. Curr. Opin. Plant Biol. 5, 543–552 (2002).
Haywood, V., Kragler, F. & Lucas, W. J. Plasmodesmata: pathways for protein and ribonucleoprotein signaling. Plant Cell 14, S303–S325 (2002).
Wu, X. L., Weigel, D. & Wigge, P. A. Signaling in plants by intercellular RNA and protein movement. Genes Dev. 16, 151–158 (2002).
Aaziz, R., Dinant, S. & Epel, B. L. Plasmodesmata and plant cytoskeleton. Trends Plant Sci. 6, 326–330 (2001).
Ehlers, K. & Kollmann, R. Primary and secondary plasmodesmata: structure, origin, and functioning. Protoplasma 216, 1–30 (2001).
Ding, B., Itaya, A. & Qi, Y. J. Symplasmic protein and RNA traffic: regulatory points and regulatory factors. Curr. Opin. Plant Biol. 6, 596–602 (2003).
Roberts, A. G. & Oparka, K. J. Plasmodesmata and the control of symplastic transport. Plant Cell Environ. 26, 103–124 (2003).
Huala, E. & Sussex, I. M. Determination and cell interactions in reproductive meristems. Plant Cell 5, 1157–1165 (1993).
Jackson, D. & Hake, S. Morphogenesis on the move: cell-to-cell trafficking of plant regulatory proteins. Curr. Opin. Genet. Dev. 7, 495–500 (1997).
Gilbertson, R. L. & Lucas, W. J. How do plant viruses traffic on the 'vascular highway'? Trends Plant Sci. 1, 260–268 (1996).
Stratmann, J. W. Long distance run in the wound response — jasmonic acid is pulling ahead. Trends Plant Sci. 8, 247–250 (2003).
Ryan, C. A., Pearce, G., Scheer, J. & Moura, D. S. Polypeptide hormones. Plant Cell 14, S251–S264 (2002).
Ruiz-Medrano, R., Xoconostle-Cázares, B. & Lucas, W. J. The phloem as a conduit for inter-organ communication. Curr. Opin. Plant Biol. 4, 202–209 (2001).
Oparka, K. J. & Santa Cruz, S. The great escape: phloem transport and unloading of macromolecules. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 323–347 (2000).
Schulz, A. Plasmodesmal widening accompanies the short-term increase in symplasmic phloem unloading in pea root-tips under osmotic-stress. Protoplasma 188, 22–37 (1995).
Gisel, A., Barella, S., Hempel, F. D. & Zambryski, P. C. Temporal and spatial regulation of symplastic trafficking during development in Arabidopsis thaliana apices. Development 126, 1879–1889 (1999).
Gisel, A., Hempel, F. D., Barella, S. & Zambryski, P. Leaf-to-shoot apex movement of symplastic tracer is restricted coincident with flowering in Arabidopsis. Proc. Natl Acad. Sci. USA 99, 1713–1717 (2002).
Crawford, K. M. & Zambryski, P. C. Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol. 125, 1802–1812 (2001).
Rinne, P. L. H. & van der Schoot, C. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development 125, 1477–1485 (1998).
Ruan, Y. L., Llewellyn, D. J. & Furbank, R. T. The control of single-celled cotton fiber elongation by developmentally reversible gating of plasmodesmata and coordinated expression of sucrose and K+ transporters and expansin. Plant Cell 13, 47–60 (2001). Elegantly shows the correlation between developmentally reversible closure of plasmodesmata — in conjunction with altered gene expression — and the elongation of cotton-fibre cells.
Crawford, K. M. & Zambryski, P. C. Subcellular localization determines the availability of non-targeted proteins to plasmodesmatal transport. Curr. Biol. 10, 1032–1040 (2000).
Boyko, V., Ferralli, J., Ashby, J., Schellenbaum, P. & Heinlein, M. Function of microtubules in intercellular transport of plant virus RNA. Nature Cell Biol. 2, 826–832 (2000).
Gillespie, T. et al. Functional analysis of a DNA-shuffled movement protein reveals that microtubules are dispensable for the cell-to-cell movement of Tobacco mosaic virus. Plant Cell 14, 1207–1222 (2003).
Kragler, F., Monzer, J., Xoconostle-Cázares, B. & Lucas, W. J. Peptide antagonists of the plasmodesmal macromolecular trafficking pathway. EMBO J. 19, 2856–2868 (2000).
Escobar, N. M. et al. High-throughput viral expression of cDNA–green fluorescent protein fusions reveals novel subcellular addresses and identifies unique proteins that interact with plasmodesmata. Plant Cell 15, 1507–1523 (2003). Used viral infection for the transient expression of random cDNA–GFP gene fusions. This approach allowed the effective screening of protein subcellular localization, which resulted in the identification of putative plasmodesma-associated proteins.
Baluska, F., Cvrckova, F., Kendrick-Jones, J. & Volkmann, D. Sink plasmodesmata as gateways for phloem unloading. Myosin VIII and calreticulin as molecular determinants of sink strength? Plant Physiol. 126, 39–46 (2001).
Blackman, L. M., Harper, J. D. I. & Overall, R. L. Localization of a centrin-like protein to higher plant plasmodesmata. Eur. J. Cell Biol. 78, 297–304 (1999).
Blackman, L. M. & Overall, R. L. Immunolocalisation of the cytoskeleton to plasmodesmata of Chara corallina. Plant J. 14, 733–741 (1998).
Murillo, I., Roca, R., Bortolotti, C. & Segundo, B. S. Engineering photoassimilate partitioning in tobacco plants improves growth and productivity and provides pathogen resistance. Plant J. 36, 330–341 (2003). Shows an intriguing relationship between alterations in assimilate allocation and the defence response against pathogens. This results from the constitutive expression of the maize PRms gene, which localizes to plasmodesmata. Also contributes to the idea that plasmodesmata have an important role in regulating plant growth and defence.
Kim, I., Hempel, F. D., Sha, K., Pfluger, J. & Zambryski, P. C. Identification of a developmental transition in plasmodesmatal function during embryogenesis in Arabidopsis thaliana. Development 129, 1261–1272 (2002). The first elegant genetic screen for Arabidopsis thaliana mutants that show an alteration in plasmodesmal function. The increased size-exclusion limit of plasmodesmata during embryogenesis and the growth defects that are observed in the ise1 and ise2 mutants show the importance of plasmodesmal function in orchestrating plant growth and development.
Ritzenthaler, C., Findlay, K., Roberts, K. & Maule, A. J. Rapid detection of plasmodesmata in purified cell walls. Protoplasma 211, 165–171 (2000).
Yahalom, A., Lando, R., Katz, A. & Epel, B. L. A calcium-dependent protein kinase is associated with maize mesocotyl plasmodesmata. J. Plant Physiol. 53, 354–362 (1998).
Blackman, L. M., Gunning, B. E. S. & Overall, R. L. A 45 kDa protein isolated from the nodal walls of Chara corallina is localised to plasmodesmata. Plant J. 15, 401–411 (1998).
Lee, J. Y. et al. Selective trafficking of non-cell-autonomous proteins mediated by NCAPP1. Science 299, 392–396 (2003). Shows the successful biochemical isolation of a new cellular factor, NCAPP1, that is involved in mediating selective trafficking of NCAPs through plasmodesmata. Provides strong support for the hypothesis that macromolecular trafficking is a regulated process and is important in cell-fate determination.
van den Berg, C., Willemsen, V., Hendriks, G., Weisbeek, P. & Scheres, B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 390, 287–289 (1997).
Benfey, P. N. & Scheres, B. Root development. Curr. Biol. 10, R813–R815 (2000).
Di Laurenzio, L. et al. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86, 423–433 (1996).
Helariutta, Y. et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101, 555–567 (2000).
Nakajima, K., Sena, G., Nawy, T. & Benfey, P. N. Intercellular movement of the putative transcription factor SHR in root patterning. Nature 413, 307–311 (2001).
Prochiantz, A. & Joliot, A. Can transcription factors function as cell–cell signalling molecules? Nature Rev. Mol. Cell Biol. 4, 814–819 (2003).
Masucci, J. D. et al. The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 122, 1253–1260 (1996).
Wada, T., Tachibana, T., Shimura, Y. & Okada, K. Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277, 1113–1116 (1997).
Schellmann, S. et al. TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. EMBO J. 21, 5036–5046 (2002).
Lee, M. M. & Schiefelbein, J. Cell pattern in the Arabidopsis root epidermis determined by lateral inhibition with feedback. Plant Cell 14, 611–618 (2002).
Wada, T. et al. Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development 129, 5409–5419 (2002). Provides clear evidence for the non-cell-autonomous role of CAPRICE (CPC ) in cell-fate determination in root-hair-cell development. Movement of CPC–GFP from non-root-hair cells into adjacent cells rescued the cpc mutant as it restored normal root-hair-cell development. Also supports the idea that macromolecular trafficking is important in plant-cell patterning.
Sultan, S. E. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 5, 537–542 (2000).
Fletcher, J. C. Shoot and floral meristem maintenance in Arabidopsis. Annu. Rev. Plant Biol. 53, 45–66 (2002).
Pruitt, R. E., Bowman, J. L. & Grossniklaus, U. Plant genetics: a decade of integration. Nature Genet. 33, 294–304 (2003).
Dievart, A. & Clark, S. E. LRR-containing receptors regulating plant development and defense. Development 131, 251–261 (2004).
Lucas, W. J. et al. Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science 270, 1980–1983 (1995).
Jackson, D., Veit, B. & Hake, S. Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120, 405–413 (1994).
Kim, J. Y., Yuan, Z. A., Cilia, M., Khalfan-Jagani, Z. & Jackson, D. Intercellular trafficking of a KNOTTED1 green fluorescent protein fusion in the leaf and shoot meristem of Arabidopsis. Proc. Natl Acad. Sci. USA 99, 4103–4108 (2002).
Jackson, D. Double labeling of KNOTTED1 mRNA and protein reveals multiple potential sites of protein trafficking in the shoot apex. Plant Physiol. 129, 1423–1429 (2002).
Kim, J. Y., Yuan, Z. & Jackson, D. Developmental regulation and significance of KNOX protein trafficking in Arabidopsis. Development 130, 4351–4362 (2003). Describes a thorough examination of the potential mechanisms underlying KNOTTED and KNOTTED-related protein movement through plasmodesmata.
Weigel, D., Alvarez, J., Smyth, D. R., Yanofsky, M. F. & Meyerowitz, E. M. Leafy controls floral meristem identity in Arabidopsis. Cell 69, 843–859 (1992).
Sessions, A., Yanofsky, M. F. & Weigel, D. Cell–cell signaling and movement by the floral transcription factors LEAFY and APETALA1. Science 289, 779–781 (2000).
Wu, X. et al. Modes of intercellular transcription factor movement in the Arabidopsis apex. Development 130, 3735–3745 (2003). Contrary to the regulated movement of KNOTTED1, LEAFY seemed to move through plasmodesmata by simple diffusion. However, LEAFY movement was not entirely unregulated as its lateral movement within cell layers was quite restricted. This is an important example indicating that control over NCAP trafficking can occur at both the plasmodesmal and NCAP levels.
Ormenese, S., Havelange, A., Bernier, G. & van der Schoot, C. The shoot apical meristem of Sinapis alba L. expands its central symplasmic field during the floral transition. Planta 215, 67–78 (2002).
Wolf, S., Deom, C. M., Beachy, R. N. & Lucas, W. J. Movement protein of tobacco mosaic virus modifies plasmodesmatal size exclusion limit. Science 246, 377–379 (1989).
Deom, C. M., Lapidot, M. & Beachy, R. N. Plant virus movement proteins. Cell 69, 221–224 (1992).
Lee, J. -Y. & Lucas, W. J. Phosphorylation of viral movement proteins — regulation of cell-to-cell trafficking. Trends Microbiol. 9, 5–8 (2001).
Karpova, O. V., Ivanov, K. I., Rodionova, N. P., Dorokhov, Y. L. & Atabekov, J. G. Nontranslatability and dissimilar behavior in plants and protoplasts of viral RNA and movement protein complexes formed in vitro. Virology 230, 11–21 (1997).
Karpova, O. V. et al. Phosphorylation of tobacco mosaic virus movement protein abolishes its translation repressing ability. Virology 261, 20–24 (1999).
Waigmann, E., Chen, M. H., Bachmaier, R., Ghoshroy, S. & Citovsky, V. Regulation of plasmodesmal transport by phosphorylation of tobacco mosaic virus cell-to-cell movement protein. EMBO J. 19, 4875–4884 (2000). Provides strong support for the involvement of phosphorylation as a mechanism to regulate NCAP trafficking through plasmodesmata. Molecular modifications made to specific TMV movement-protein phosphorylation sites blocked systemic infection and rendered the movement protein incompetent for cell-to-cell movement. Interestingly, this regulatory effect of movement-protein phosphorylation was host dependent, occurring in some, but not other, species of tobacco.
Foo, E., Turnbull, C. G. N. & Beveridge, C. A. Long-distance signaling and the control of branching in the rms1 mutant of pea. Plant Physiol. 126, 203–209 (2001).
Beveridge, C. A., Weller, J. L., Singer, S. R. & Hofer, J. M. I. Axillary meristem development. Budding relationships between networks controlling flowering, branching, and photoperiod responsiveness. Plant Physiol. 131, 927–934 (2003).
Lucas, W. J., Yoo, B. -C. & Kragler, F. RNA as a long-distance information macromolecule in plants. Nature Rev. Mol. Cell Biol. 2, 849–857 (2001).
van Bel, A. J. E. Transport phloem: low profile, high impact. Plant Physiol. 131, 1509–1510 (2003).
Xoconostle-Cázares, B. et al. Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 283, 94–98 (1999).
Owens, R. A., Blackburn, M. & Ding, B. Possible involvement of the phloem lectin in long-distance viroid movement. Mol. Plant Microbe Interact. 14, 905–909 (2001).
Balachandran, S., Xiang, Y., Schobert, C., Thompson, G. A. & Lucas, W. J. Phloem sap proteins from Cucurbita maxima and Ricinus communis have the capacity to traffic cell to cell through plasmodesmata. Proc. Natl Acad. Sci. USA 94, 14150–14155 (1997).
Imlau, A., Truernit, E. & Sauer, N. Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell 11, 309–322 (1999).
Zambryski, P. Cell-to-cell transport of proteins and fluorescent tracers via plasmodesmata during plant development. J. Cell Biol. 164, 165–168 (2004).
Oparka K. J. et al. Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 97, 743–754 (1999).
Itaya, A. et al. Plasmodesma-mediated selective protein traffic between 'symplasmically isolated' cells probed by a viral movement protein. Plant Cell 14, 2071–2083 (2002). Elegantly shows that NCAP movement can occur beyond the symplasmic boundary/domain established by the CC–PP plasmodesmata. Importantly, it it shows that NCAP trafficking can occur between the phloem and its surrounding tissues, and therefore supports the concept that long-distance translocation of NCAPs can regulate physiological and developmental processes.
Ishiwatari, Y. et al. Rice phloem thioredoxin h has the capacity to mediate its own cell-to-cell transport through plasmodesmata. Planta 205, 12–22 (1998).
Xoconostle-Cázares, B., Ruiz-Medrano, R. & Lucas, W. J. Proteolytic processing of CmPP36, a protein from the cytochrome b5 reductase family, is required for entry into the phloem translocation pathway. Plant J. 24, 735–747 (2000).
Hoffmann-Benning, S., Gage, D. A., McIntosh, L., Kende, H. & Zeevaart, J. A. D. Comparison of peptides in the phloem sap of flowering and non-flowering Perilla and lupine plants using microbore HPLC followed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Planta 216, 140–147 (2002).
Yoo, B. C., Lee, J. Y. & Lucas, W. J. Analysis of the complexity of protein kinases within the phloem sieve tube system — characterization of Cucurbita maxima calmodulin-like domain protein kinase 1. J. Biol. Chem. 277, 15325–15332 (2002).
Yoo, B. C. et al. Characterization of Cucurbita maxima phloem serpin-1 (CmPS-1). A developmentally regulated elastase inhibitor. J. Biol. Chem. 275, 35122–35128 (2000).
Aoki, K., Kragler, F., Xoconostle-Cázares, B. & Lucas, W. J. A subclass of plant heat shock cognate 70 chaperones carries a motif that facilitates trafficking through plasmodesmata. Proc. Natl Acad. Sci. USA 99, 16342–16347 (2002). Reports the identification of the first motif that potentiates the cell-to-cell movement of the NCAP HSPc70. This motif was not found in cytosolic non-phloem HSP70s, but its transfer to a human HSP70 resulted in a gain of function. This work implicated a role for phloem-mobile HSPc70s chaperones in mediating NCAP trafficking though CC–SE plasmodesmata.
Fisher, D. B. & Cash-Clark, C. E. Sieve tube unloading and post-phloem transport of fluorescent tracers and proteins injected into sieve tubes via severed aphid stylets. Plant Physiol. 123, 125–137 (2000).
Jorgensen, R. A. RNA traffics information systemically in plants. Proc. Natl Acad. Sci. USA 99, 11561–11563 (2002).
Ruiz-Medrano, R., Xoconostle-Cázares, B. & Lucas, W. J. Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development 126, 4405–4419 (1999).
Foster, T. M. et al. A surveillance system regulates selective entry of RNA into the shoot apex. Plant Cell 14, 1497–1508 (2002). Provides the provocative finding that entry of RNA into the shoot apex might be controlled by an RNA-surveillance system. Transgenic plants expressing a potexvirus movement protein were compromised in their ability to exclude both viral RNA and silencing signals from the shoot apex, resulting in defects in organ development.
Chen, J. -J., Janssen, B. J., Williams, A. & Sinha, N. A. A gene fusion at a homeobox locus: alterations in leaf shape and implications for morphological evolution. Plant Cell 9, 1289–1304 (1997).
Kim, M., Canio, W., Kessler, S. & Sinha, N. Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 293, 287–289 (2001). Used grafting techniques to provide the first evidence of a functional role for mRNA that is translocated through phloem. Graft transmission of the Me mutant transcripts into wild-type scion meristems resulted in the development of organs displaying the mutant phenotype.
Hannon, G. J. RNA interference. Nature 418, 244–251 (2002).
Mlotshwa, S. RNA silencing and the mobile silencing signal. Plant Cell 14, S289–S301 (2002).
Palauqui, J. -C., Elmayan, T., Pollien, J. -M. & Vaucheret, H. Systemic acquired silencing: Transgene-specific post-transcriptional silencing is transmitted by grafting from silenced stocks to non-silenced scions. EMBO J. 16, 4738–4745 (1997).
Voinnet, O., Vain, P., Angell, S. & Baulcombe, D. C. Systemic spread of sequence-specific transgene RNA degradation in plants is initiated by localized introduction of ectopic promoterless DNA. Cell 95, 177–187 (1998).
Ruiz, M. T., Voinnet, O. & Baulcombe, D. C. Initiation and maintenance of virus-induced gene silencing. Plant Cell 10, 937–946 (1998).
Hamilton, A., Voinnet, O., Chappell, L. & Baulcombe, D. Two classes of short interfering RNA in RNA silencing. EMBO J. 21, 4671–4679 (2002).
Mallory, A. C., Mlotshwa, S., Bowman, L. H. & Vance, V. B. The capacity of transgenic tobacco to send a systemic RNA silencing signal depends on the nature of the inducing transgene locus. Plant J. 35, 82–92 (2003).
Klahre, U., Crété, P., Leuenberger, S. A., Iglesias, V. A. & Meins, F. High molecular weight RNAs and small interfering RNAs induce systemic posttranscriptional gene silencing in plants. Proc. Natl Acad. Sci. USA 99, 11981–11986 (2002).
Sticher, L., Mauch-Mani, B. & Metraux, J. P. Systemic acquired resistance. Annu. Rev. Phytopathol. 35, 235–270 (1997).
Veronese, P. et al. In defense against pathogens: both plant sentinels and foot soldiers need to know the enemy. Plant Physiol. 131, 1580–1590 (2003).
Scheer, J. M. & Ryan, C. A. The systemin receptor SR160 from Lycopersicon peruvianum is a member of the LRR receptor kinase family. Proc. Natl Acad Sci. USA 99, 9585–9590 (2002). Reports on the isolation of a 160-kDa systemin cell-surface receptor (SR160) from the plant plasma membrane and its identification as a member of the leucine-rich repeat (LRR) receptor-kinase family; interestingly, SR160 is closely related to the brassinolide receptor kinase, BRI1.
Navaez-Vasquez, J. & Ryan, C. A. The cellular localization of prosystemin: a functional role for phloem parenchyma in systemic wound signaling. Planta 218, 360–369 (2004).
Li, L., Li, C. Y., Lee, G. I. & Howe, G. A. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc. Natl Acad. Sci. USA 99, 6416–6421 (2002).
Lee, G. I. & Howe, G. A. The tomato mutant spr1 is defective in systemin perception and the production of a systemic wound signal for defense gene expression. Plant J. 33, 567–576 (2003). Used an spr1 mutant, which was previously identified as a suppressor of prosystemin-mediated responses, to show that systemin functions at, or near, the site of wounding to increase jasmonate synthesis to a level required for a systemic wound response. Implies the existence of a systemin-independent pathway for wound signalling.
Hause, B., Hause, G., Kutter, C., Miersch, O. & Wasternack, C. Enzymes of jasmonate biosynthesis occur in tomato sieve elements. Plant Cell Physiol. 44, 643–648 (2003).
Schultze, M. & Kondorosi, A. Regulation of symbiotic root nodule development. Annu. Rev. Genet. 32, 33–57 (1998).
Varma Penmetsa, R., Frugoli, J. A., Smith, L., Long, S. R. & Cook, D. R. Dual genetic pathways controlling nodule number in Medicago truncatula. Plant Physiol. 131, 998–1008 (2003).
Searle, I. R. et al. Long-distance signaling in nodulation directed by a CLAVATA 1-like receptor kinase. Science 299, 109–112 (2003). The first example of successful positional cloning from soybean providing intriguing molecular insight into the autoregulation of nodule formation. NARK (nodule-autoregulation receptor kinase), which is closely related to the meristem stem-cell-suppression gene CLAVATA1 of A. thaliana , was shown to function in the long-distance nodule-suppression signal pathway.
Complainville, A. et al. Nodule initiation involves the creation of a new symplasmic field in specific root cells of Medicago species. Plant Cell 15, 2778–2791 (2003). Outstanding demonstration of the creation of a symplasmic field between the SE–CC and nodule initials, at an early stage in nodule formation in the root.
Lucas, W. J. & Wolf, S. Plasmodesmata: the intercellular organelles of green plants. Trends Cell Biol. 3, 308–315 (1993).
Radford J. E. & White R. G. Localization of a myosin-like protein to plasmodesmata. Plant J. 14, 743–750 (1998).
Van Gestel K. et al. Immunological evidence for the presence of plant homologues of the actin-related protein Arp3 in tobacco and maize: subcellular localization to actin-enriched pit fields and emerging root hairs. Protoplasma 222, 45–52 (2003).
Perbal, M. C., Haughn, G., Saedler, H. & Schwarz-Sommer, Z. Non-cell-autonomous function of the Antirrhinum floral homeotic proteins DEFICIENS and GLOBOSA is exerted by their polar cell-to-cell trafficking. Development 122, 3433–3441 (1996).
Hantke, S. S., Carpenter, R. & Coen, E. S. Expression of floricaula in single cell layers of periclinal chimeras activates downstream homeotic genes in all layers of floral meristems. Development 121, 27–35 (1995).
Durfee, T. et al. F-box-containing protein UFO and AGAMOUS participate in antagonistic pathways governing early petal development in Arabidopsis. Proc. Natl Acad. Sci. USA 100, 8571–8576 (2003).
Acknowledgements
Our thanks go to colleagues who provided unpublished work for inclusion in this review and especially to J. Bowman, B. Scheres and T.-S. Yu for providing unpublished photomicrographs. We apologize to all those whose work could not be discussed due to space limitations. Work in our laboratory on plasmodesmata and the supracellular nature of plants is supported by grants from the Department of Energy Office of Basic Energy Sciences and the National Science Foundation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- CELL–CELL COMMUNICATION
-
Signalling that occurs through the release of a ligand into the extracellular milieu. From there, it can diffuse and bind to its membrane-bound receptor to activate a specific signal cascade within the target cell.
- NUCLEAR PORE COMPLEX
-
A large multiprotein complex that forms a channel in the nuclear envelope of eukaryotic cells. It joins the inner and outer nuclear membranes and allows transport of proteins and nucleic acids to and from the nucleus.
- METABOLITES
-
Small molecules (100–500 Da), such as simple sugars, organic acids and amino acids, that participate in biochemical and/or physiological processes that occur within the cell.
- SYMPLASMIC DOMAIN
-
The confinement of molecular exchange by a cytoplasmic continuum to a discrete field of cells.
- GAP JUNCTION
-
Communicating junction (normally permeant to molecules of up to 3 kDa) between adjacent cells. It is composed of 12 connexin protein subunits — each of the coupled cells contributes a connexon, or hemichannel, that is formed from 6 connexin subunits.
- CELL-TO-CELL COMMUNICATION
-
Signalling that involves the direct movement of an information molecule into the cytoplasm of neighbouring cells without entry into the extracellular milieu. This pathway involves gap junctions in animals and plasmodesmata in plants and algae.
- RIBONUCLEOPROTEIN (RNP) COMPLEX
-
A complex of protein and RNA. In many cases, the proteins can recognize their cognate mRNA molecules (selective binding) and mediate their delivery to specific regions within the cell.
- SUPRACELLULAR ORGANISMS
-
Organs of these complex organisms consist of populations of cells, but almost every cell is interconnected by plasmodesmata to its nearest neighbours. As the phloem interconnects all symplasmic domains in the plant, such a system of organization works above the level of the cell, hence the term 'supracellular'.
- NON-CELL-AUTONOMOUS PROTEINS (NCAPS)
-
Plant proteins that move between cells using either the cell–cell or cell-to-cell pathway. Examples include small ligands that diffuse through the extracellular milieu and bind to receptors that are located in the plasma membrane, and transcription factors that enter the nuclei of neighbouring cells to participate in cell-fate determination.
- NON-CELL-AUTONOMOUS PROTEIN PATHWAY
-
(NCAP pathway). The route along which NCAPs move between cells. The components of this pathway can include structural elements within the cytoplasm (for example, the cytoskeleton), carriers/chaperones, plasmodesmal docking proteins, structural constituents of the plasmodesmata and regulatory proteins (such as protein kinases).
- MICROINJECTION
-
A method that is used to introduce a range of test molecules into the cytoplasm of living cells. In plants, this technique has provided a powerful tool to explore, directly, the capacity of a protein (or fluorescent molecule) to be transported from cell to cell through plasmodesmata.
- GREEN FLUORESCENT PROTEIN
-
A heterologous protein from Aequorea victoria (jellyfish) that is used to study NCAP trafficking through plasmodesmata. It is also used to trace the movement of a tagged protein both within the cell and during intercellular trafficking.
- INFLORESCENCE STEM
-
The stem that carries the flowers.
- SHOOT APICAL MERISTEM
-
The vegetative apex of a plant consists of a group of stem cells (or initials) that form the shoot apical meristem, together with the lateral organs — leaf primordia, vascular initials, axillary buds and the stem — that are derived from these cells.
- MOVEMENT PROTEIN
-
A virally-encoded protein that binds to viral RNA/DNA to mediate the cell-to-cell trafficking of the infectious component of the viral genome. Endogenous NCAPs can also be referred to as movement proteins.
- PROTOPLAST
-
An osmotically sensitive plant cell that lacks its cell wall but still has its plasma membrane.
- XYLEM
-
Vascular tissue that delivers water and mineral nutrients, which are taken up by the root system, to vegetative organs. It also provides mechanical support.
- PHLOEM
-
A component of the plant vascular system that delivers sugars, amino acids, mineral nutrients and information molecules (hormones, peptide hormones, proteins and RNA) to developing tissues and organs. Translocation occurs from source tissues to tissues that require nutrients to support growth. Transport occurs through a unique sieve-tube system, is unidirectional, and is driven by a gradient of positive pressure.
- COMPANION CELL
-
A specialized cell that is associated with a sieve element in the phloem of flowering plants.
- SIEVE ELEMENT
-
A cell of the phloem that functions in the long-distance transport of sugars and signalling molecules.
- SYMPLASM
-
A cytoplasmic continuum that occurs between cells of a tissue or organ. It is mediated by plasmodesmata or gap junctions.
- SYSTEMIC ACQUIRED RESISTANCE (SAR)
-
Resistance to pathogens that occurs when defence signalling molecules are transmitted through the phloem to upregulate specific defence genes.
- MONOCOTYLEDON
-
A plant that has a single cotyledon (seed leaf) as an embryo.
- PRIMORDIA
-
Plants form appendages, such as leaves, flowers and lateral roots, through the generation of groups of cells that collectively are called primordia. Cells that are incorporated into these developmental domains are ultimately derived from the shoot or root apical meristems.
Rights and permissions
About this article
Cite this article
Lucas, W., Lee, JY. Plasmodesmata as a supracellular control network in plants. Nat Rev Mol Cell Biol 5, 712–726 (2004). https://doi.org/10.1038/nrm1470
Issue Date:
DOI: https://doi.org/10.1038/nrm1470
This article is cited by
-
Silver nanoparticle ecotoxicity and phytoremediation: a critical review of current research and future prospects
Environmental Science and Pollution Research (2024)
-
Plasmodesmata mediate cell-to-cell transport of brassinosteroid hormones
Nature Chemical Biology (2023)
-
A review on phytotoxicity and defense mechanism of silver nanoparticles (AgNPs) on plants
Journal of Nanoparticle Research (2023)
-
Silver nanoparticles improved morphogenesis, biochemical profile and micro-morphology of Gaillardia pulchella Foug cv. ‘Torch Yellow’
Plant Cell, Tissue and Organ Culture (PCTOC) (2023)
-
Examining the evidence for extracellular RNA function in mammals
Nature Reviews Genetics (2021)