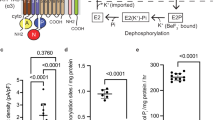
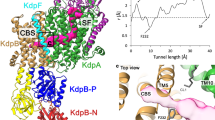
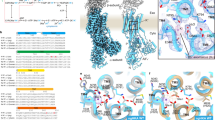
Key Points
-
P-type ATPases use the energy from ATP hydrolysis to pump ions across the cell membrane against a concentration gradient.
-
They form a large family of ubiquitous membrane proteins, and carry out many essential processes, such as generating the membrane potential or removing toxic ions from cells.
-
P-type ATPases undergo large conformational changes in the ion-pumping cycle. Recent X-ray and electron-microscopy structures of P-type ATPases in different conformations have provided the first detailed insights into the mechanism of ATP-driven ion translocation.
-
The common structural elements of all P-type ATPases are four protein domains, each with highly conserved features, which indicates that they all share the same basic mechanism.
-
The 10 membrane-spanning α-helices of the membrane domain form the ion-translocation site and provide a mechanical link to the three cytoplasmic domains that carry out ATP hydrolysis
-
The activity of most P-type ATPases is tightly controlled by extra regulatory domains or protein subunits. The regulatory mechanisms are poorly understood.
Abstract
P-type ATPases are ion pumps that carry out many fundamental processes in biology and medicine, ranging from the generation of membrane potential to muscle contraction and the removal of toxic ions from cells. Making use of the energy stored in ATP, they transport specific ions across the cell membrane against a concentration gradient. Recent X-ray structures and homology models of P-type pumps now provide a basis for understanding the molecular mechanism of ATP-driven ion transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Skou, J. C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim. Biophys. Acta 23, 394–401 (1957). The first description of an ATP-driven ion pump, which is now known as the Na+/K+-ATPase. A paper worth a Nobel prize 40 years later.
Hasselbach, W. & Makinose, M. Die Calciumpumpe der Erschlaffungsgrana des Muskels und ihre Abhängigkeit von der ATP-Spaltung. Eur. J. Biochem. 333, 518–528 (1961).
Slayman, C. L., Lu, C. Y. & Shane, L. Correlated changes in membrane potential and ATP concentrations in Neurospora. Nature 226, 274–276 (1970).
Jorgensen, P. L. Purification and characterization of (Na+,K+)-ATPase V. Conformational changes in the enzyme. Transitions between the Na-form and the K-form studied with tryptic digestion as a tool. Biochim. Biophys. Acta 401, 399–415 (1975).
Jencks, W. P. Utilization of binding energy and coupling rules for active transport and other coupled vectorial processes. Methods Enzymol. 171, 145–164 (1989).
Moller, J. V., Juul, B. & le Maire, M. Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim. Biophys. Acta 1, 1–51 (1996).
Axelsen, K. B. & Palmgren, M. G. Evolution of substrate specificities in the P-type ATPase superfamily. J. Mol. Evol. 46, 84–101 (1998). A thorough analysis of the sequence homology among P-type ATPases.
Bult, C. J. et al. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science 273, 1058–1073 (1996).
Goffeau, A. The inventory of all ion and drug ATPases encoded by the yeast genome. Acta Physiol. Scand. 643, 297–300 (1998).
Axelsen, K. B. & Palmgren, M. G. Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol. 126, 696–706 (2001).
Okamura, H., Yasuhara, J. C., Fambrough, D. M. & Takeyasu, K. P-type ATPases in Caenorhabditis and Drosophila: implications for evolution of the P-type ATPase subunit families with special references to the Na,K-ATPase and H,K-ATPase subgroup. J. Membr. Biol. 191, 13–24 (2002).
Palmgren, M. G. & Axelsen, K. B. Evolution of P-type ATPases. Biochim. Biophys. Acta 1365, 37–45 (1998).
Altendorf, K. et al. Structure and function of the Kdp-ATPase of Escherichia coli. Acta Physiol. Scand. 643, 137–146 (1998).
Rensing, C., Fan, B., Sharma, R., Mitra, B. & Rosen, B. P. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc. Natl Acad. Sci. USA 97, 652–656 (2000).
Okkeri, J. & Haltia, T. Expression and mutagenesis of ZntA, a zinc-transporting P-type ATPase from Escherichia coli. Biochemistry 38, 14109–14116 (1999).
Rosen, B. P. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp. Biochem. Physiol., Part A Mol. Integr. Physiol. 133, 689–693 (2002).
Nelson, N. Metal ion transporters and homeostasis. EMBO J. 18, 4361–4371 (1999).
Lutsenko, S. & Petris, M. J. Function and regulation of the mammalian copper-transporting ATPases: insights from biochemical and cell biological approaches. J. Membr. Biol. 191, 1–12 (2003).
Tanzi, R. E. et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nature Genet. 5, 344–350 (1993).
Odermatt, A., Suter, H., Krapf, R. & Solioz, M. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J. Biol. Chem. 268, 12775–12779 (1993).
Bull, P. C., Thomas, G. R., Rommens, J. M., Forbes, J. R. & Cox, D. W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nature Genet. 5, 327–337 (1993).
Daleke, D. L. Regulation of transbilayer plasma membrane phospholipd asymmetry. J. Lipid Res. 44, 233–242 (2003).
Pomorski, T. et al. Drs2p-related-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell 14, 1240–1254 (2003).
Daleke, D. L. & Huestis, W. H. Erythrocyte morphology reflects the transbilayer distribution of incorporated phospholipids. J. Cell Biol. 108, 1375–1385 (1989).
Lee, A. G. A calcium pump made visible. Curr. Opin. Struct. Biol. 12, 547–554 (2002).
Stokes, D. L. & Green, N. M. Structure and function of the calcium pump. Annu. Rev. Biophys. Biomol. Struct. 32, 445–468 (2003). A clear and authoritative review on the SR Ca2+-ATPase, which puts a large amount of data into context and proposes a structure-based mechanism for the catalytic cycle.
Toyoshima, C., Nakasako, M., Nomura, H. & Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 405, 647–655 (2000). The first X-ray structure of a P-type ATPase defines the four principal domains, and highlights a surprisingly long distance between the ion-translocation site in the membrane and the cytoplasmic phosphorylation site.
Toyoshima, C. & Nomura, H. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418, 605–611 (2002). The 3.1-Å X-ray structure of SR Ca2+-ATPase trapped in an E2 state indicates significant rigid-body movements of the three cytoplasmic domains, as well as helix movements in the membrane that result in the disruption of the ion-binding site and Ca2+ release to the outside medium.
Yu, X., Carroll, S., Rigaud, J. L. & Inesi, G. H+ countertransport and electrogenicity of the sarcoplasmic reticulum Ca2+ pump in reconstituted proteoliposomes. Biophys. J. 64, 1232–1242 (1993).
Ma, J. J. & Pan, Z. Junctional membrane structure and store operated calcium entry in muscle cells. Front. Biosci. 8, D242–D255 (2003).
Harper, J. F. Dissecting calcium oscillators in plant cells. Trends Plant Sci. 6, 395–397 (2001).
Palmgren, M. G. Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Mol. Biol. 52, 817–845 (2001). All there is to know about the plant proton pump — an excellent, comprehensive review.
Geisler, M., Koenen, W., Richter, J. & Schumann, J. Molecular aspects of higher plant P-type Ca2+-ATPases. Biochim. Biophys. Acta 1456, 52–78 (2000).
Kaplan, J. H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535 (2002).
Jorgensen, P., Hakansson, K. & Karlish, S. Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu. Rev. Physiol. 65, 817–849 (2003). A detailed, authoritative review of the Na+/K+-ATPase, which puts a large body of biochemical and biophysical data into a structural context.
Mense, M., Rajendran, V., Blostein, R. & Caplan, M. J. Extracellular domains, transmembrane segments, and intracellular domains interact to determine the cation selectivity of Na,K- and gastric H,K-ATPase. Biochemistry 41, 9803–9812 (2002).
Vagin, O., Denevich, S., Munson, K. & Sachs, G. SCH28080, a K+-competitive inhibitor of the gastric H,K-ATPase, binds near the M5–6 luminal loop, preventing K+ access to the ion binding domain. Biochemistry 41, 12755–12762 (2002).
Monk, B. C. & Perlin, D. S. Fungal plasma membrane proton pumps as promising new antifungal targets. Crit. Rev. Microbiol. 20, 209–223 (1994).
Besancon, M. et al. Membrane topology and omeprazole labeling of the gastric H+,K(+)-adenosinetriphosphatase. Biochemistry 32, 2345–2355 (1993).
Geering, K. The functional role of β subunits in oligomeric P-type ATPases. J. Bioenerg. Biomembr. 33, 425–438 (2001).
Therien, A. G., Pu, H. X., Karlish, S. J. & Blostein, R. Molecular and functional studies of the γ subunit of the sodium pump. J. Bioenerg. Biomembr. 33, 407–414 (2001).
Geering, K. et al. FXYD proteins: new tissue- and isoform-specific regulators of Na,K-ATPase. Ann. NY Acad. Sci. 986, 388–394 (2003).
Morsomme, P. et al. Characterization of a hyperthermophilic P-type ATPase from Methanococcus jannaschii expressed in yeast. J. Biol. Chem. 277, 29608–29616 (2002).
Eraso, P. & Gancedo, C. Activation of yeast plasma membrane ATPase by acid pH during growth. FEBS Lett. 224, 187–192 (1987).
Skriver, E., Maunsbach, A. B. & Jorgensen, P. L. Formation of two-dimensional crystals in pure membrane-bound (Na+K+)-ATPase. FEBS Lett. 131, 219–222 (1981).
Dux, L. & Martonosi, A. Two-dimensional arrays of proteins in sarcoplasmic reticulum and purified Ca2+-ATPase vesicles treated with vanadate. J. Biol. Chem. 258, 2599–2603 (1983).
Rabon, E., Wilke, M., Sachs, G. & Zampighi, G. Crystallization of the gastric H,K-ATPase. J. Mol. Biol. 261, 1434–1439 (1986).
Xu, C., Rice, W. J., He, W. & Stokes, D. L. A structural model for the catalytic cycle of Ca2+-ATPase. J. Mol. Biol. 316, 201–211 (2002). A 6-Å map of the vanadate-inhibited SR Ca2+-ATPase indicates a central role for the A-domain in the catalytic cycle.
Rice, W. J. et al. Structure of Na+,K+-ATPase at 11-Å resolution: comparison with Ca2+-ATPase in E1 and E2 states. Biophys. J. 80, 2187–2197 (2001). The best structure of a Na+/K+-ATPase so far highlights a close similarity to the SR Ca2+-ATPase in the E2–P state.
Auer, M., Scarborough, G. A. & Kühlbrandt, W. Three-dimensional map of the plasma membrane H+-ATPase in the open conformation. Nature 392, 840–843 (1998). The first, and so far only, structure of an H+-pump in an E1 state, which was determined to an 8-Å resolution by electron crystallography.
Jahn, T. et al. Large scale expression, purification and 2D crystallization of recombinant plant plasma membrane H+-ATPase. J. Mol. Biol. 309, 465–476 (2001).
Zhang, P., Toyoshima, C., Yonekura, K., Green, N. M. & Stokes, D. L. Structure of the calcium pump from sarcoplasmic reticulum at 8 Å resolution. Nature 392, 835–839 (1998).
Lahiri, S. D., Zhang, G., Dunaway-Mariano, D. & Allen, K. N. The pentacovalent phosphorus intermediate of a phosphoryl transfer reaction. Science 299, 2067–2071 (2003). The 1.2-Å structure of a close homologue of the P-type-ATPase P-domain shows the crucial Asp residue in the process of being phosphorylated.
Hilge, M. et al. ATP-induced conformational changes of the nucleotide-binding domain of Na,K-ATPase. Nature Struct. Biol. 10, 468–474 (2003). The solution NMR structure of a recombinant N-domain shows the triphosphate of ATP protruding from the nucleotide-binding pocket.
Kühlbrandt, W., Zeelen, J. & Dietrich, J. Structure, mechanism, and regulation of the Neurospora plasma membrane H+-ATPase. Science 297, 1692–1696 (2002). A homology model of the N. crassa H+-pump highlights a significant difference in the position of the N-domain compared with the SR Ca2+-ATPase. In addition, the position of the carboxy-terminal R-domain of the H+-pump indicates a structure-based mechanism of enzyme regulation.
Ogawa, H. & Toyoshima, C. Homology modeling of the cation binding sites of Na+K+-ATPase. Proc. Natl Acad. Sci. USA 99, 15977–15982 (2002).
Aravind, L., Galperin, M. Y. & Koonin, E. V. The catalytic domain of the P-type ATPase has the haloacid dehalogenase fold. Trends Biochem. Sci. 23, 127–129 (1998).
Toyofuku, T., Kurzydlowski, K., Tada, M. & MacLennan, D. H. Amino acids Lys-Asp-Asp-Lys-Pro-Val402 in the Ca(2+)-ATPase of cardiac sarcoplasmic reticulum are critical for functional association with phospholamban. J. Biol. Chem. 269, 22929–22932 (1994).
Patchornik, G., Goldshleger, R. & Karlish, S. J. The complex ATP–Fe2+ serves as a specific affinity cleavage reagent in ATP–Mg2+ sites of Na,K-ATPase: altered ligation of Fe2+ (Mg2+) ions accompanies the E1P→E2P conformational change. Proc. Natl Acad. Sci. USA 97, 11954–11959 (2000).
Gitschier, J., Moffat, B., Reilly, D., Wood, W. I. & Fairbrother, W. J. Solution structure of the fourth metal-binding domain from the Menkes copper-transporting ATPase. Nature Struct. Biol. 5, 47–54 (1998).
Banci, L. et al. A new zinc-protein coordination site in intracellular metal trafficking: solution structure of the Apo and Zn(II) forms of ZntA(46–118). J. Mol. Biol. 323, 883–897 (2002).
Hill, E. E., Morea, V. & Chothia, C. Sequence conservation in families whose members have little or no sequence similarity: the four-helical cytokines and cytochromes. J. Mol. Biol. 322, 205–233 (2002).
Baldwin, J. M. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 12, 1693–1703 (1993).
Buch-Pedersen, M. J. et al. Abolishment of proton pumping and accumulation in the E1P conformational state of a plant plasma membrane H+-ATPase by substitution of a conserved aspartyl residue in transmembrane segment 6. J. Biol. Chem. 275, 39167–39173 (2000).
Buch-Pedersen, M. J. & Palmgren, M. G. Conserved Asp684 in transmembrane segment M6 of the plant plasma membrane P-type proton pump AHA2 is a molecular determinant of proton translocation. J. Biol. Chem. 278, 17845–17851 (2003).
Bukrinsky, J. T., Buch-Pedersen, M. J., Larsen, S. & Palmgren, M. G. A putative proton binding site of plasma membrane H(+)-ATPase identified through homology modelling. FEBS Lett. 494, 6–10 (2001).
Danko, S., Yamasaki, K., Daiho, T., Suzuki, H. & Toyoshima, C. Organization of cytoplasmic domains of sarcoplasmic reticulum Ca2+-ATPase in E1P and E1ATP states: a limited proteolysis study. FEBS Lett. 505, 129–135 (2001).
Wakabayashi, S. & Shigekawa, M. Role of divalent cation bound to phosphoenzyme intermediate of sarcoplasmic reticulum ATPase. J. Biol. Chem. 259, 4427–4436 (1984).
Stokes, D. L. & Green, N. M. Modeling a dehalogenase fold into the 8-Å density map for Ca(2+)-ATPase defines a new domain structure. Biophys. J. 78, 1765–1776 (2000).
Portillo, F. Regulation of plasma membrane H(+)-ATPase in fungi and plants. Biochim. Biophys. Acta 1469, 31–42 (2000).
Petris, M. J. et al. Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J. 15, 6084–6095 (1996).
East, J. M. Sarco(endo)plasmic reticulum calcium pumps: recent advances in our understanding of structure/function and biology. Mol. Membr. Biol. 17, 189–200 (2000).
MacLennan, D. H. & Kranias, E. G. Phospholamban: a crucial regulator of cardiac contractility. Nature Rev. Mol. Cell Biol. 4, 566–577 (2003).
Toyoshima, C. et al. Modeling of the inhibitory interaction of phospholamban with the Ca2+ ATPase. Proc. Natl Acad. Sci. USA 100, 467–472 (2003).
Carafoli, E. Biogenesis: plasma membrane calcium ATPase: 15 years of work on the purified enzyme. FASEB J. 8, 993–1002 (1994).
Curran, A. C. et al. Autoinhibition of a calmodulin-dependent calcium pump involves a structure in the stalk that connects the transmembrane domain to the ATPase catalytic domain. J. Biol. Chem. 275, 30301–30308 (2000).
Sze, H., Liang, F., Hwang, I., Curran A. C. & Harper J. F. Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu. Rev. Plant Physiol. Mol. Biol. 51, 433–462 (2000).
Palmgren, M. G., Sommarin, M., Serrano, R. & Larsson, C. Identification of an autoinhibitory domain in the C-terminal region of the plant plasma membrane H(+)-ATPase. J. Biol. Chem. 266, 20470–20475 (1991).
Palmgren, M. Regulation of plant plasma-membrane H+-ATPase activity. Physiol. Plantarum 83, 314–323 (1991).
Serrano, R., Portillo, F., Monk, B. C. & Palmgren, M. G. The regulatory domain of fungal and plant plasma membrane H(+)-ATPase. Acta Physiol. Scand. Suppl. 607, 131–136 (1992).
Morsomme, P., Slayman, C. W. & Goffeau, A. Mutagenic study of the structure, function and biogenesis of the yeast plasma membrane H(+)-ATPase. Biochim. Biophys. Acta 1469, 133–157 (2000).
Oecking, C., Piotrowski, M., Hagemeier, J. & Hagemann, K. Topology of the fusicoccin-binding 14-3-3 homologs of Commelina communis. Plant J. 12, 441–453 (1997).
Fuglsang, A. T. et al. Binding of 14-3-3 protein to the plasma membrane H(+)-ATPase AHA2 involves the three C-terminal residues Tyr(946)-Thr-Val and requires phosphorylation of Thr(947). J. Biol. Chem. 274, 36774–36780 (1999).
Aducci, P., Marra, M., Fogliano, V. & Fullone M. R. Fusicoccin receptors: perception and transduction of the fusicoccin-signal. J. Exp. Bot. 46, 1463–1478 (1995).
Würtele, M., Jelich-Ottmann, C., Wittinghofer, A. & Oecking, C. Structural view of a fungal toxin acting on a 14-3-3 regulatory complex. EMBO J. 22, 987–994 (2003). An X-ray structure of a complex of the last five residues of the plant H+-ATPase R-domain with a 14-3-3 protein shows how a fungal toxin activates this proton pump.
Goossens, A., de la Fuente, N., Forment, J., Serrano, R. & Portillo, F. Regulation of yeast H+-ATPase by protein kinases belonging to a family dedicated to activation of plasma membrane transporters. Mol. Cell. Biol. 20, 7654–7661 (2000).
Portillo, F., Eraso, P. & Serrano, R. Analysis of the regulatory domain of yeast plasma membrane H+-ATPase by directed mutagenesis and intragenic suppression. FEBS Lett. 287, 71–74 (1991).
Goormaghtigh, E., Vigneron, L., Scarborough, G. A. & Ruysschaert, J. M. Tertiary conformational changes of the Neurospora crassa plasma membrane H(+)-ATPase monitored by hydrogen/deuterium exchange kinetics. A Fourier transformed infrared spectroscopy approach. J. Biol. Chem. 269, 27409–27413 (1994).
Rhee, K. H., Scarborough, G. A. & Henderson, R. Domain movements of plasma membrane H(+)-ATPase: 3D structures of two states by electron cryo-microscopy. EMBO J. 21, 3582–3589 (2002).
Geibel, S., Kaplan, J. H., Bamberg, E. & Freidrich, T. Conformational dynamics of the Na+/K+-ATPase probed by voltage clamp fluorometry. Proc. Natl Acad. Sci. USA 100, 964–969 (2003).
Zhou, Y., Morais-Cabral, J. H., Kaufman, A. & MacKinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel–Fab complex at 2.0 Å resolution. Nature 414, 43–48 (2001).
Deisenhofer, J. & Michel, H. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. Biosci. Rep. 9, 383–419 (1989).
Rhee, K. H. Photosystem II: the solid structural era. Annu. Rev. Biophys. Biomol. Struct. 30, 307–328 (2001).
Doyle, D. A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998).
Jiang, Y. et al. X-ray structure of a voltage-dependent K+ channel. Nature 423, 33–41 (2003).
Albers, R. Biochemical aspects of active transport. Annu. Rev. Biochem. 36, 727–756 (1967).
Post, R. L., Hegyvary, C. & Kume, S. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J. Biol. Chem. 247, 6530–6540 (1972).
Henderson, R. et al. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J. Mol. Biol. 213, 899–929 (1990).
Kühlbrandt, W., Wang, D. N. & Fujiyoshi, Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature 367, 614–621 (1994).
Miyazawa, A., Fujiyoshi, Y. & Unwin, N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 423, 949–955 (2003).
Chothia, C. & Lesk, A. M. The relation between the divergence of sequence and structure in proteins. EMBO J. 5, 823–826 (1986).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Thompson, J. D., Higgins, D. G. & Gibson, T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680 (1994).
Sali, A. & Blundell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Acknowledgements
I thank Gitte Mohsin and Paolo Lastrico for preparing the figures, and Mickey Palmgren for many helpful hints.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Entrez
Protein Data Bank
Glossary
- MEMBRANE POTENTIAL
-
The charge difference (measured in mV) between the two surfaces of a biological membrane that arises from the different concentrations of ions such as H+, Na+ or K+ on either side. The Na+/K+-ATPase creates a membrane potential by using the energy stored in ATP to maintain a low concentration of Na+ and a high concentration of K+ in the cell, against a higher concentration of Na+ and a lower concentration of K+ on the outside.
- FXYD PROTEIN FAMILY
-
A small family of short, single-span membrane proteins that contain the FXYD sequence motif (in which X can be any amino acid). Most known FXYD proteins regulate the activity of Na+/K+-ATPases in particular tissues. For example, the FXYD protein phospholemman regulates Na+/K+-ATPases in heart and skeletal muscle, and the γ-subunit, another FXYD protein, regulates renal Na+/K+-ATPase.
- ROSSMANN FOLD
-
A common structural motif that is found in the nucleotide-binding domains of many proteins. The typical Rossmann fold (named after the eminent protein crystallographer Michael Rossmann) consists of two structurally similar halves, each with three β-strands and two α-helices. The two halves are connected by a linking helix, and form a compact, globular α/β domain with a central, six-stranded parallel β-sheet.
- 14-3-3 PROTEINS
-
A large class of proteins that are involved in cell division, apoptosis, signal transduction, transmitter release, receptor function, gene expression and enzyme activation in eukaryotes. They function by binding to a wide range of different, specific target proteins, usually in response to phosphorylation of these targets.
Rights and permissions
About this article
Cite this article
Kühlbrandt, W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol 5, 282–295 (2004). https://doi.org/10.1038/nrm1354
Issue Date:
DOI: https://doi.org/10.1038/nrm1354
This article is cited by
-
Involvement of reactive oxygen species in zinc-deficiency induced inhibition of crown root growth in maize plant
Plant and Soil (2024)
-
Whole genome sequencing of the halophilic Halomonas qaidamensis XH36, a novel species strain with high ectoine production
Antonie van Leeuwenhoek (2022)
-
Structural and functional comparison of magnesium transporters throughout evolution
Cellular and Molecular Life Sciences (2022)
-
Microbial silver resistance mechanisms: recent developments
World Journal of Microbiology and Biotechnology (2022)
-
Structure and transport mechanism of P5B-ATPases
Nature Communications (2021)