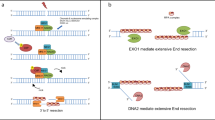
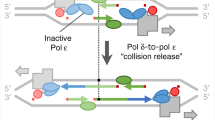
Key Points
-
Ribonucleotides are incorporated into DNA during replication.
-
Ribonucleotides can be removed during ribonucleotide excision repair or topoisomerase I-initiated processing.
-
Failure of ribonucleotide removal is associated with genome instability in the form of mutagenesis, replication stress, DNA breaks and chromosomal rearrangements.
-
Human diseases, including autoimmune disorders and neurodegenerative diseases, may be associated with failure to process genomic ribonucleotides
-
Genomic ribonucleotides function as a strand-discrimination signal during DNA mismatch repair, and they may have other physiological roles.
Abstract
The information encoded in DNA is influenced by the presence of non-canonical nucleotides, the most frequent of which are ribonucleotides. In this Review, we discuss recent discoveries about ribonucleotide incorporation into DNA during replication by the three major eukaryotic replicases, DNA polymerases α, δ and ε. The presence of ribonucleotides in DNA causes short deletion mutations and may result in the generation of single- and double-strand DNA breaks, leading to genome instability. We describe how these ribonucleotides are removed from DNA through ribonucleotide excision repair and by topoisomerase I. We discuss the biological consequences and the physiological roles of ribonucleotides in DNA, and consider how deficiencies in their removal from DNA may be important in the aetiology of disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kuchta, R. D. & Stengel, G. Mechanism and evolution of DNA primases. Biochim. Biophys. Acta 1804, 1180–1189 (2010).
Martinez-Jimenez, M. I. et al. Alternative solutions and new scenarios for translesion DNA synthesis by human PrimPol. DNA Repair 29, 127–138 (2015).
Goodman, M. F. & Woodgate, R. Translesion DNA polymerases. Cold Spring Harb. Perspect. Biol. 5, a010363 (2013).
Boiteux, S. & Jinks-Robertson, S. DNA repair mechanisms and the bypass of DNA damage in Saccharomyces cerevisiae. Genetics 193, 1025–1064 (2013).
Sollier, J. & Cimprich, K. A. Breaking bad: R-loops and genome integrity. Trends Cell Biol. 25, 514–522 (2015).
Santos-Pereira, J. M. & Aguilera, A. R loops: new modulators of genome dynamics and function. Nat. Rev. Genet. 16, 583–597 (2015).
Jinks-Robertson, S. & Bhagwat, A. S. Transcription-associated mutagenesis. Annu. Rev. Genet. 48, 341–359 (2014).
Brown, J. A. & Suo, Z. Unlocking the sugar “steric gate” of DNA polymerases. Biochemistry 50, 1135–1142 (2011).
Joyce, C. M. Choosing the right sugar: how polymerases select a nucleotide substrate. Proc. Natl Acad. Sci. USA 94, 1619–1622 (1997).
Yeeles, J. T., Poli, J., Marians, K. J. & Pasero, P. Rescuing stalled or damaged replication forks. Cold Spring Harb. Perspect. Biol. 5, a012815 (2013).
Wan, L. et al. hPrimpol1/CCDC111 is a human DNA primase-polymerase required for the maintenance of genome integrity. EMBO Rep. 14, 1104–1112 (2013).
Garcia-Gomez, S. et al. PrimPol, an archaic primase/polymerase operating in human cells. Mol. Cell 52, 541–553 (2013).
Bianchi, J. et al. PrimPol bypasses UV photoproducts during eukaryotic chromosomal DNA replication. Mol. Cell 52, 566–573 (2013).
Mouron, S. et al. Repriming of DNA synthesis at stalled replication forks by human PrimPol. Nat. Struct. Mol. Biol. 20, 1383–1389 (2013).
Kennedy, E. M., Amie, S. M., Bambara, R. A. & Kim, B. Frequent incorporation of ribonucleotides during HIV-1 reverse transcription and their attenuated repair in macrophages. J. Biol. Chem. 287, 14280–14288 (2012).
Nick McElhinny, S. A. et al. Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc. Natl Acad. Sci. USA 107, 4949–4954 (2010).
Traut, T. W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 140, 1–22 (1994).
Ferraro, P., Franzolin, E., Pontarin, G., Reichard, P. & Bianchi, V. Quantitation of cellular deoxynucleoside triphosphates. Nucleic Acids Res. 38, e85 (2010).
Kumar, D., Viberg, J., Nilsson, A. K. & Chabes, A. Highly mutagenic and severely imbalanced dNTP pools can escape detection by the S-phase checkpoint. Nucleic Acids Res. 38, 3975–3983 (2010).
Kumar, D. et al. Mechanisms of mutagenesis in vivo due to imbalanced dNTP pools. Nucleic Acids Res. 39, 1360–1371 (2011).
Chabes, A. et al. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell 112, 391–401 (2003).
Buckland, R. J. et al. Increased and imbalanced dNTP pools symmetrically promote both leading and lagging strand replication infidelity. PLoS Genet. 10, e1004846 (2014).
Davidson, M. B. et al. Endogenous DNA replication stress results in expansion of dNTP pools and a mutator phenotype. EMBO J. 31, 895–907 (2012).
Williams, L. N. et al. dNTP pool levels modulate mutator phenotypes of error-prone DNA polymerase ε variants. Proc. Natl Acad. Sci. USA 112, E2457–E2466 (2015).
Mertz, T. M., Sharma, S., Chabes, A. & Shcherbakova, P. V. Colon cancer-associated mutator DNA polymerase δ variant causes expansion of dNTP pools increasing its own infidelity. Proc. Natl Acad. Sci. USA 112, E2467–E2476 (2015).
Watt, D. L., Buckland, R. J., Lujan, S. A., Kunkel, T. A. & Chabes, A. Genome-wide analysis of the specificity and mechanisms of replication infidelity driven by imbalanced dNTP pools. Nucleic Acids Res. 44, 1669–1680 (2015).
Shinbrot, E. et al. Exonuclease mutations in DNA polymerase epsilon reveal replication strand specific mutation patterns and human origins of replication. Genome Res. 24, 1740–1750 (2014).
Church, D. N. et al. DNA polymerase ε and δ exonuclease domain mutations in endometrial cancer. Hum. Mol. Genet. 22, 2820–2828 (2013).
Yoshida, R. et al. Concurrent genetic alterations in DNA polymerase proofreading and mismatch repair in human colorectal cancer. Eur. J. Hum. Genet. 19, 320–325 (2011).
The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 (2012).
Palles, C. et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat. Genet. 45, 136–144 (2013).
Kandoth, C. et al. Integrated genomic characterization of endometrial carcinoma. Nature 497, 67–73 (2013).
Clausen, A. R., Zhang, S., Burgers, P. M., Lee, M. Y. & Kunkel, T. A. Ribonucleotide incorporation, proofreading and bypass by human DNA polymerase δ. DNA Repair 12, 121–127 (2013).
Goksenin, A. Y. et al. Human DNA polymerase ε is able to efficiently extend from multiple consecutive ribonucleotides. J. Biol. Chem. 287, 42675–42684 (2012).
Williams, J. S. et al. Evidence that processing of ribonucleotides in DNA by topoisomerase 1 is leading-strand specific. Nat. Struct. Mol. Biol. 22, 291–297 (2015).
Nick McElhinny, S. A. et al. Genome instability due to ribonucleotide incorporation into DNA. Nat. Chem. Biol. 6, 774–781 (2010). Shows that ribonucleotides are incorporated into yeast genomic DNA and, together with reference 42, that mis-incorporated ribonucleotides can be removed by RNase H2.
Williams, J. S. et al. Proofreading of ribonucleotides inserted into DNA by yeast DNA polymerase ε. DNA Repair 11, 649–656 (2012).
Clausen, A. R. et al. Tracking replication enzymology in vivo by genome-wide mapping of ribonucleotide incorporation. Nat. Struct. Mol. Biol. 22, 185–191 (2015).
Koh, K. D., Balachander, S., Hesselberth, J. R. & Storici, F. Ribose-seq: global mapping of ribonucleotides embedded in genomic DNA. Nat. Methods 12, 251–257 (2015).
Cerritelli, S. M. & Crouch, R. J. Ribonuclease H: the enzymes in eukaryotes. FEBS J. 276, 1494–1505 (2009).
Eder, P. S., Walder, R. Y. & Walder, J. A. Substrate specificity of human RNase H1 and its role in excision repair of ribose residues misincorporated in DNA. Biochimie 75, 123–126 (1993).
Rydberg, B. & Game, J. Excision of misincorporated ribonucleotides in DNA by RNase H (type 2) and FEN-1 in cell-free extracts. Proc. Natl Acad. Sci. USA 99, 16654–16659 (2002).
Lujan, S. A. et al. Mismatch repair balances leading and lagging strand DNA replication fidelity. PLoS Genet. 8, e1003016 (2012).
Reijns, M. A. et al. Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell 149, 1008–1022 (2012).
Hiller, B. et al. Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. J. Exp. Med. 209, 1419–1426 (2012).
Cavanaugh, N. A. et al. Molecular insights into DNA polymerase deterrents for ribonucleotide insertion. J. Biol. Chem. 286, 31650–31660 (2011).
Wang, W., Wu, E. Y., Hellinga, H. W. & Beese, L. S. Structural factors that determine selectivity of a high fidelity DNA polymerase for deoxy-, dideoxy-, and ribonucleotides. J. Biol. Chem. 287, 28215–28226 (2012).
Kasiviswanathan, R. & Copeland, W. C. Ribonucleotide discrimination and reverse transcription by the human mitochondrial DNA polymerase. J. Biol. Chem. 286, 31490–31500 (2011).
DeLucia, A. M., Grindley, N. D. & Joyce, C. M. An error-prone family Y DNA polymerase (DinB homolog from Sulfolobus solfataricus) uses a 'steric gate' residue for discrimination against ribonucleotides. Nucleic Acids Res. 31, 4129–4137 (2003).
Lujan, S. A., Williams, J. S., Clausen, A. R., Clark, A. B. & Kunkel, T. A. Ribonucleotides are signals for mismatch repair of leading-strand replication errors. Mol. Cell 50, 437–443 (2013).
Kunkel, T. A. & Burgers, P. M. Dividing the workload at a eukaryotic replication fork. Trends Cell Biol. 18, 521–527 (2008).
Miyabe, I., Kunkel, T. A. & Carr, A. M. The major roles of DNA polymerases epsilon and delta at the eukaryotic replication fork are evolutionarily conserved. PLoS Genet. 7, e1002407 (2011).
Daigaku, Y. et al. A global profile of replicative polymerase usage. Nat. Struct. Mol. Biol. 22, 192–198 (2015).
Jinks-Robertson, S. & Klein, H. L. Ribonucleotides in DNA: hidden in plain sight. Nat. Struct. Mol. Biol. 22, 176–178 (2015).
Williams, J. S. & Kunkel, T. A. Ribonucleotides in DNA: origins, repair and consequences. DNA Repair 19, 27–37 (2014).
Shcherbakova, P. V. et al. Unique error signature of the four-subunit yeast DNA polymerase ε. J. Biol. Chem. 278, 43770–43780 (2003).
Johnson, R. E., Klassen, R., Prakash, L. & Prakash, S. A. Major role of DNA polymerase δ in replication of both the leading and lagging DNA strands. Mol. Cell 59, 163–175 (2015).
Flood, C. L. et al. Replicative DNA polymerase δ but not ε proofreads errors in cis and in trans. PLoS Genet. 11, e1005049 (2015).
Sparks, J. L. et al. RNase H2-initiated ribonucleotide excision repair. Mol. Cell 47, 980–986 (2012).
Chon, H. et al. Contributions of the two accessory subunits, RNASEH2B and RNASEH2C, to the activity and properties of the human RNase H2 complex. Nucleic Acids Res. 37, 96–110 (2009).
Bubeck, D. et al. PCNA directs type 2 RNase H activity on DNA replication and repair substrates. Nucleic Acids Res. 39, 3652–3666 (2011).
Kind, B. et al. Altered spatio-temporal dynamics of RNase H2 complex assembly at replication and repair sites in Aicardi–Goutieres syndrome. Hum. Mol. Genet. 23, 5950–5960 (2014).
Cai, Y., Geacintov, N. E. & Broyde, S. Ribonucleotides as nucleotide excision repair substrates. DNA Repair 13, 55–60 (2014).
Vaisman, A. et al. Removal of misincorporated ribonucleotides from prokaryotic genomes: an unexpected role for nucleotide excision repair. PLoS Genet. 9, e1003878 (2013).
Lindsey-Boltz, L. A., Kemp, M. G., Hu, J. & Sancar, A. Analysis of ribonucleotide removal from DNA by human nucleotide excision repair. J. Biol. Chem. 290, 29801–29807 (2015).
Williams, J. S. et al. Topoisomerase 1-mediated removal of ribonucleotides from nascent leading-strand DNA. Mol. Cell 49, 1010–1015 (2013).
Pizzi, S. et al. Reduction of hRNase H2 activity in Aicardi–Goutieres syndrome cells leads to replication stress and genome instability. Hum. Mol. Genet. 24, 649–658 (2015).
Chon, H. et al. RNase H2 roles in genome integrity revealed by unlinking its activities. Nucleic Acids Res. 41, 3130–3143 (2013). Demonstrates that the functions of RNase H2 can be unlinked, and establishes that short deletion mutagenesis caused by unrepaired genomic ribonucleotides is due to failure to remove single ribonucleotides from DNA.
Kim, N. et al. Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science 332, 1561–1564 (2011). Shows that the short deletion mutagenesis associated with loss of RNase H2 activity in yeast is caused by Top1 cleavage at a ribonucleotide.
Sekiguchi, J. & Shuman, S. Site-specific ribonuclease activity of eukaryotic DNA topoisomerase I. Mol. Cell 1, 89–97 (1997).
Conover, H. N. et al. Stimulation of chromosomal rearrangements by ribonucleotides. Genetics 201, 951–961 (2015).
Ashour, M. E., Atteya, R. & El-Khamisy, S. F. Topoisomerase-mediated chromosomal break repair: an emerging player in many games. Nat. Rev. Cancer 15, 137–151 (2015).
Lippert, M. J. et al. Role for topoisomerase 1 in transcription-associated mutagenesis in yeast. Proc. Natl Acad. Sci. USA 108, 698–703 (2011).
Takahashi, T., Burguiere-Slezak, G., Van der Kemp, P. A. & Boiteux, S. Topoisomerase 1 provokes the formation of short deletions in repeated sequences upon high transcription in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 108, 692–697 (2011).
Cho, J. E., Kim, N., Li, Y. C. & Jinks-Robertson, S. Two distinct mechanisms of topoisomerase 1-dependent mutagenesis in yeast. DNA Repair 12, 205–211 (2013).
Cho, J. E., Kim, N. & Jinks-Robertson, S. Topoisomerase 1-dependent deletions initiated by incision at ribonucleotides are biased to the non-transcribed strand of a highly activated reporter. Nucleic Acids Res. 43, 9306–9313 (2015).
Yu, H. & Droge, P. Replication-induced supercoiling: a neglected DNA transaction regulator? Trends Biochem. Sci. 39, 219–220 (2014).
Caldecott, K. W. Ribose — an internal threat to DNA. Science 343, 260–261 (2014).
DeRose, E. F., Perera, L., Murray, M. S., Kunkel, T. A. & London, R. E. Solution structure of the Dickerson DNA dodecamer containing a single ribonucleotide. Biochemistry 51, 2407–2416 (2012).
Koh, K. D., Chiu, H. C., Riedo, E. & Storici, F. Measuring the elasticity of ribonucleotide(s)-containing DNA molecules using AFM. Methods Mol. Biol. 1297, 43–57 (2015).
Jaishree, T. N., van der Marel, G. A., van Boom, J. H. & Wang, A. H. Structural influence of RNA incorporation in DNA: quantitative nuclear magnetic resonance refinement of d(CG)r(CG)d(CG) and d(CG)r(C)d(TAGCG). Biochemistry 32, 4903–4911 (1993).
Egli, M., Usman, N. & Rich, A. Conformational influence of the ribose 2′-hydroxyl group: crystal structures of DNA–RNA chimeric duplexes. Biochemistry 32, 3221–3237 (1993).
Ban, C., Ramakrishnan, B. & Sundaralingam, M. A single 2′-hydroxyl group converts B-DNA to A-DNA — crystal structure of the DNA–RNA chimeric decamer duplex d(CCGGC)R(CCGG) with a novel intermolecular G·C base-paired quadruplet. J. Mol. Biol. 236, 275–285 (1994).
Rychlik, M. P. et al. Crystal structures of RNase H2 in complex with nucleic acid reveal the mechanism of RNA–DNA junction recognition and cleavage. Mol. Cell 40, 658–670 (2010).
Chiu, H. C. et al. RNA intrusions change DNA elastic properties and structure. Nanoscale 6, 10009–10017 (2014).
Li, Y. & Breaker, R. R. Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2′-hydroxyl group. J. Am. Chem. Soc. 121, 5326–5372 (1999).
Alvey, H. S., Gottardo, F. L., Nikolova, E. N. & Al-Hashimi, H. M. Widespread transient Hoogsteen base pairs in canonical duplex DNA with variable energetics. Nat. Commun. 5, 4786 (2014).
Dunn, K. & Griffith, J. D. The presence of RNA in a double helix inhibits its interaction with histone protein. Nucleic Acids Res. 8, 555–566 (1980).
Hovatter, K. R. & Martinson, H. G. Ribonucleotide-induced helical alteration in DNA prevents nucleosome formation. Proc. Natl Acad. Sci. USA 84, 1162–1166 (1987).
Chen, J. Z., Qiu, J., Shen, B. & Holmquist, G. P. Mutational spectrum analysis of RNase H(35) deficient Saccharomyces cerevisiae using fluorescence-based directed termination PCR. Nucleic Acids Res. 28, 3649–3656 (2000). Demonstrates that the spontaneous mutation spectrum of an RNase H2-deficient budding yeast strain is dominated by a 4 bp deletion.
Clark, A. B., Lujan, S. A., Kissling, G. E. & Kunkel, T. A. Mismatch repair-independent tandem repeat sequence instability resulting from ribonucleotide incorporation by DNA polymerase ε. DNA Repair 10, 476–482 (2011).
Sparks, J. L. & Burgers, P. M. Error-free and mutagenic processing of topoisomerase 1-provoked damage at genomic ribonucleotides. EMBO J. 34, 1259–1269 (2015).
Huang, S. Y., Ghosh, S. & Pommier, Y. Topoisomerase I alone is sufficient to produce short DNA deletions and can also reverse nicks at ribonucleotide sites. J. Biol. Chem. 290, 14068–14076 (2015). Together with reference 92, shows that Top1 processing of genomic ribonucleotides can be either error-free or mutagenic, and biochemically defines these pathways.
Potenski, C. J., Niu, H., Sung, P. & Klein, H. L. Avoidance of ribonucleotide-induced mutations by RNase H2 and Srs2-Exo1 mechanisms. Nature 511, 251–254 (2014).
Gambus, A. et al. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat. Cell Biol. 8, 358–366 (2006).
Wu, J., Phatnani, H. P., Hsieh, T. S. & Greenleaf, A. L. The phosphoCTD-interacting domain of Topoisomerase I. Biochem. Biophys. Res. Commun. 397, 117–119 (2010).
Watt, D. L., Johansson, E., Burgers, P. M. & Kunkel, T. A. Replication of ribonucleotide-containing DNA templates by yeast replicative polymerases. DNA Repair 10, 897–902 (2011).
Clausen, A. R., Murray, M. S., Passer, A. R., Pedersen, L. C. & Kunkel, T. A. Structure-function analysis of ribonucleotide bypass by B family DNA replicases. Proc. Natl Acad. Sci. USA 110, 16802–16807 (2013).
Storici, F., Bebenek, K., Kunkel, T. A., Gordenin, D. A. & Resnick, M. A. RNA-templated DNA repair. Nature 447, 338–341 (2007).
Lazzaro, F. et al. RNase H and postreplication repair protect cells from ribonucleotides incorporated in DNA. Mol. Cell 45, 99–110 (2012).
Ulrich, H. D. Timing and spacing of ubiquitin-dependent DNA damage bypass. FEBS Lett. 585, 2861–2867 (2011).
Tumbale, P., Williams, J. S., Schellenberg, M. J., Kunkel, T. A. & Williams, R. S. Aprataxin resolves adenylated RNA–DNA junctions to maintain genome integrity. Nature 506, 111–115 (2014). Demonstrates that abortive DNA ligation occurs at RNase H2 incision sites in DNA to generate an adenylated 5′ end that requires the APTX deadenylase for repair.
Moreira, M. C. et al. The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat. Genet. 29, 189–193 (2001).
Gao, R. et al. Proteolytic degradation of topoisomerase II (Top2) enables the processing of Top2·DNA and Top2·RNA covalent complexes by tyrosyl-DNA-phosphodiesterase 2 (TDP2). J. Biol. Chem. 289, 17960–17969 (2014).
Andres, S. N., Schellenberg, M. J., Wallace, B. D., Tumbale, P. & Williams, R. S. Recognition and repair of chemically heterogeneous structures at DNA ends. Environ. Mol. Mutagen. 56, 1–21 (2015).
Wallace, B. D. & Williams, R. S. Ribonucleotide triggered DNA damage and RNA–DNA damage responses. RNA Biol. 11, 1340–1346 (2014).
Aguilera, A. & Klein, H. L. Genetic control of intrachromosomal recombination in Saccharomyces cerevisiae. I. Isolation and genetic characterization of hyper-recombination mutations. Genetics 119, 779–790 (1988).
Anand, R. P., Lovett, S. T. & Haber, J. E. Break-induced DNA replication. Cold Spring Harb. Perspect. Biol. 5, a010397 (2013).
Wahba, L., Amon, J. D., Koshland, D. & Vuica-Ross, M. RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol. Cell 44, 978–988 (2011).
Allen-Soltero, S., Martinez, S. L., Putnam, C. D. & Kolodner, R. D. A Saccharomyces cerevisiae RNase H2 interaction network functions to suppress genome instability. Mol. Cell. Biol. 34, 1521–1534 (2014).
Ii, M., Ii, T., Mironova, L. I. & Brill, S. J. Epistasis analysis between homologous recombination genes in Saccharomyces cerevisiae identifies multiple repair pathways for Sgs1, Mus81–Mms4 and RNase H2. Mutat. Res. 714, 33–43 (2011).
O'Connell, K., Jinks-Robertson, S. & Petes, T. D. Elevated genome-wide instability in yeast mutants lacking RNase H activity. Genetics 201, 963–975 (2015).
Crow, Y. J. et al. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi–Goutieres syndrome and mimic congenital viral brain infection. Nat. Genet. 38, 910–916 (2006). Reports a genetic association between mutations in RNase H2 and the rare neuroinflammatory disorder AGS.
Reijns, M. A. & Jackson, A. P. Ribonuclease H2 in health and disease. Biochem. Soc. Trans. 42, 717–725 (2014).
Reijns, M. A. et al. The structure of the human RNase H2 complex defines key interaction interfaces relevant to enzyme function and human disease. J. Biol. Chem. 286, 10530–10539 (2011).
Figiel, M. et al. The structural and biochemical characterization of human RNase H2 complex reveals the molecular basis for substrate recognition and Aicardi–Goutieres syndrome defects. J. Biol. Chem. 286, 10540–10550 (2011).
Gunther, C. et al. Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J. Clin. Invest. 125, 413–424 (2015). Reports a genetic association between mutations in RNase H2 and the autoimmune disease SLE, and links defective ribonucleotide removal to enhanced photosensitivity and immune signalling.
Schellenberg, M. J., Tumbale, P. P. & Williams, R. S. Molecular underpinnings of Aprataxin RNA/DNA deadenylase function and dysfunction in neurological disease. Progress Biophys. Mol. Biol. 117, 157–165 (2015).
Akbari, M., Sykora, P. & Bohr, V. A. Slow mitochondrial repair of 5′-AMP renders mtDNA susceptible to damage in APTX deficient cells. Sci. Rep. 5, 12876 (2015).
Potenski, C. J. & Klein, H. L. How the misincorporation of ribonucleotides into genomic DNA can be both harmful and helpful to cells. Nucleic Acids Res. 42, 10226–10234 (2014).
Donigan, K. A., McLenigan, M. P., Yang, W., Goodman, M. F. & Woodgate, R. The steric gate of DNA polymerase ι regulates ribonucleotide incorporation and deoxyribonucleotide fidelity. J. Biol. Chem. 289, 9136–9145 (2014).
Makarova, A. V., Nick McElhinny, S. A., Watts, B. E., Kunkel, T. A. & Burgers, P. M. Ribonucleotide incorporation by yeast DNA polymerase ζ. DNA Repair 18, 63–67 (2014).
Sastre-Moreno, G., Sanchez, A., Esteban, V. & Blanco, L. ATP insertion opposite 8-oxo-deoxyguanosine by Pol4 mediates error-free tolerance in Schizosaccharomyces pombe. Nucleic Acids Res. 42, 9821–9837 (2014).
Nick McElhinny, S. A. & Ramsden, D. A. Polymerase mu is a DNA-directed DNA/RNA polymerase. Mol. Cell. Biol. 23, 2309–2315 (2003).
Cilli, P., Minoprio, A., Bossa, C., Bignami, M. & Mazzei, F. Formation and repair of mismatches containing ribonucleotides and oxidized bases at repeated DNA sequences. J. Biol. Chem. 290, 26259–26269 (2015).
Bergoglio, V., Ferrari, E., Hubscher, U., Cazaux, C. & Hoffmann, J. S. DNA polymerase β can incorporate ribonucleotides during DNA synthesis of undamaged and CPD-damaged DNA. J. Mol. Biol. 331, 1017–1023 (2003).
Donigan, K. A. et al. Unlocking the steric gate of DNA polymerase η leads to increased genomic instability in Saccharomyces cerevisiae. DNA Repair 35, 1–12 (2015).
Gosavi, R. A., Moon, A. F., Kunkel, T. A., Pedersen, L. C. & Bebenek, K. The catalytic cycle for ribonucleotide incorporation by human DNA Pol λ. Nucleic Acids Res. 40, 7518–7527 (2012).
Vengrova, S. & Dalgaard, J. Z. RNase-sensitive DNA modification(s) initiates S. pombe mating-type switching. Genes Dev. 18, 794–804 (2004).
Vengrova, S. & Dalgaard, J. Z. The wild-type Schizosaccharomyces pombe mat1 imprint consists of two ribonucleotides. EMBO Rep. 7, 59–65 (2006). Introduces the concept of genomic ribonucleotides providing an important developmental signal.
Sayrac, S., Vengrova, S., Godfrey, E. L. & Dalgaard, J. Z. Identification of a novel type of spacer element required for imprinting in fission yeast. PLoS Genet. 7, e1001328 (2011).
Dalgaard, J. Z. Causes and consequences of ribonucleotide incorporation into nuclear DNA. Trends Genet. 28, 592–597 (2012).
Reijns, M. A. et al. Lagging-strand replication shapes the mutational landscape of the genome. Nature 518, 502–506 (2015).
Ding, J., Taylor, M. S., Jackson, A. P. & Reijns, M. A. Genome-wide mapping of embedded ribonucleotides and other noncanonical nucleotides using emRiboSeq and EndoSeq. Nat. Protoc. 10, 1433–1444 (2015).
Ghodgaonkar, M. M. et al. Ribonucleotides misincorporated into DNA act as strand-discrimination signals in eukaryotic mismatch repair. Mol. Cell 50, 323–332 (2013). Together with reference 50, demonstrates the ability of ribonucleotides to function as a strand-discrimination signal during MMR of replication errors introduced during leading strand DNA synthesis.
Kunkel, T. A. & Erie, D. A. Eukaryotic mismatch repair in relation to DNA replication. Annu. Rev. Genet. 49, 291–313 (2015).
Arana, M. E. et al. Transcriptional responses to loss of RNase H2 in Saccharomyces cerevisiae. DNA Repair 11, 933–941 (2012).
Burgers, P. M. Polymerase dynamics at the eukaryotic DNA replication fork. J. Biol. Chem. 284, 4041–4045 (2009).
Zheng, L. & Shen, B. Okazaki fragment maturation: nucleases take centre stage. J. Mol. Cell. Biol. 3, 23–30 (2011).
Balakrishnan, L. & Bambara, R. A. Okazaki fragment metabolism. Cold Spring Harb. Perspect. Biol. 5, a010173 (2013).
Garg, P., Stith, C. M., Sabouri, N., Johansson, E. & Burgers, P. M. Idling by DNA polymerase δ maintains a ligatable nick during lagging-strand DNA replication. Genes Dev. 18, 2764–2773 (2004).
Qiu, J., Qian, Y., Frank, P., Wintersberger, U. & Shen, B. Saccharomyces cerevisiae RNase H(35) functions in RNA primer removal during lagging-strand DNA synthesis, most efficiently in cooperation with Rad27 nuclease. Mol. Cell. Biol. 19, 8361–8371 (1999).
Levikova, M. & Cejka, P. The Saccharomyces cerevisiae Dna2 can function as a sole nuclease in the processing of Okazaki fragments in DNA replication. Nucleic Acids Res. 43, 7888–7897 (2015).
Kang, Y. H., Lee, C. H. & Seo, Y. S. Dna2 on the road to Okazaki fragment processing and genome stability in eukaryotes. Crit. Rev. Biochem. Mol. Biol. 45, 71–96 (2010).
Boule, J. B., Rougeon, F. & Papanicolaou, C. Terminal deoxynucleotidyl transferase indiscriminately incorporates ribonucleotides and deoxyribonucleotides. J. Biol. Chem. 276, 31388–31393 (2001).
Yang, M. Y. et al. Biased incorporation of ribonucleotides on the mitochondrial L-strand accounts for apparent strand-asymmetric DNA replication. Cell 111, 495–505 (2002).
Grossman, L. I., Watson, R. & Vinograd, J. The presence of ribonucleotides in mature closed-circular mitochondrial DNA. Proc. Natl Acad. Sci. USA 70, 3339–3343 (1973).
Shen, Y., Koh, K. D., Weiss, B. & Storici, F. Mispaired rNMPs in DNA are mutagenic and are targets of mismatch repair and RNases H. Nat. Struct. Mol. Biol. 19, 98–104 (2012).
Acknowledgements
The authors thank Dmitry Gordenin and Scott Williams for critical reading of the manuscript. The laboratory is supported by the Intramural Research Program of the US National Institutes of Health, National Institute of Environmental Health Sciences. The authors apologize to colleagues whose primary research articles are not cited owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- DNA polymerases
-
Enzymes that synthesize chains of deoxyribonucleotides.
- Translesion DNA synthesis
-
(TLS). DNA synthesis that occurs across a template DNA lesion using a specialized DNA polymerase.
- R-loops
-
Three-stranded structures that include both an RNA–DNA hybrid and a single DNA strand. R-loops are formed during transcription when the nascent mRNA hybridizes with the complementary template DNA strand and, if not removed, can threaten genome stability or regulate gene expression.
- Topoisomerase I
-
(Top1). A type 1B topoisomerase that relieves both positive and negative DNA supercoils by generating a reversible single-strand DNA nick in duplex DNA. Top1 functions during both DNA replication and transcription.
- Okazaki fragment
-
A short DNA fragment synthesized by DNA polymerase α (Pol α) and Pol δ with a length determined by nucleosome periodicity. Following removal of the RNA primer (synthesized by Pol α) to initiate synthesis, these segments are joined by DNA ligase I to form the continuous lagging strand.
- Proofreading
-
The 3′–5′ exonuclease activity possessed by some DNA polymerases that facilitates the removal of DNA mismatches before synthesis continues.
- Mismatch repair
-
(MMR). A post-replication repair process that removes base–base mismatches and deletions resulting from DNA synthesis errors. MMR occurs in a strand-specific manner and involves error recognition, excision and gap re-synthesis.
- RNase H2
-
A conserved heterotrimeric enzyme that cleaves the RNA portion of a DNA–RNA hybrid, hydrolysing both single and multiple consecutive ribonucleotides in DNA.
- B family DNA polymerases
-
A family of related DNA polymerases that are found in all domains of cellular life. The family includes the highly accurate eukaryotic and archaeal replicases, and the polymerases from phages T4 and RB69. Some possess a 3′–5′ exonucleolytic proofreading activity.
- Strand displacement synthesis
-
An activity possessed by some DNA polymerases that involves displacement of the downstream DNA to produce a flap.
- Proliferating cell nuclear antigen
-
(PCNA). A homotrimeric sliding clamp complex that plays an essential part at the replication fork through recruitment of many enzymes required for DNA replication and repair. Post-translational modifications of PCNA initiate specific DNA repair processes that are crucial for maintaining genome stability.
- Replication stress
-
A cascade of responses that result from difficulties during DNA synthesis, such as polymerase stalling at a DNA lesion in the template strand. Replication stress may slow replisome progression and sensitizes cells to exogenous replication stress-inducing agents.
- Micronuclei
-
Aberrant nuclear structures located within the cytoplasm that are composed of chromosomal fragments that were not properly incorporated into a daughter nucleus during cell division. Micronuclei are indicative of DNA damage.
- γH2AX foci
-
Nuclear foci that serve as biomarkers for DNA double-strand breaks (DSBs), formed by a variant of histone H2A that is phosphorylated at Ser139 by checkpoint kinases during DNA damage response activation and modified over a large region of chromatin in the vicinity of a DSB.
- CMG complex
-
(Cdc45/Mcm2-7/GINS complex). The helicase that unwinds DNA at eukaryotic replication forks. It is physically associated with the leading strand DNA polymerase Pol ε.
- Post-replication DNA repair
-
A process that occurs at sites of DNA damage to facilitate the completion of replication using error-free (MMS2-dependent template switching) or error-prone (DNA polymerase ζ-dependent translesion synthesis) DNA damage-tolerance pathways.
- RNA–DNA damage
-
Genome instability caused by the presence of ribonucleotides in DNA. RNA–DNA damage is associated with mutagenesis, chromosomal rearrangements, replication stress and DNA breaks.
- Gene conversion events
-
DNA repair events that involve the transfer of genetic information from a donor sequence to a homologous recipient, such that the two become identical.
- Loss of heterozygosity
-
(LOH). A chromosomal rearrangement in diploid cells that occurs when the template for DNA synthesis during homologous recombination repair is a homologous chromosome. This may result in loss of a functional allele and occurs frequently in tumour cells.
- Mitotic interhomologue recombination
-
Mitotic crossover recombination that occurs between homologous chromosomes during the repair of a DNA lesion.
- Non-allelic homologous recombination
-
(NAHR). A form of homologous recombination that occurs between similar sequences, causing chromosomal translocations and copy number variation.
- DNA fragile site
-
A specific genomic locus that is susceptible to spontaneous DNA breaks, which may result in chromosomal rearrangements and contribute to human disease.
- G4 quadruplex
-
A higher-order DNA structural motif composed of four strands of guanine-rich sequences that form a stable planar stacked structure at specific genomic locations, including telomeres and gene promoters, where they seem to have important positive and negative biological roles.
Rights and permissions
About this article
Cite this article
Williams, J., Lujan, S. & Kunkel, T. Processing ribonucleotides incorporated during eukaryotic DNA replication. Nat Rev Mol Cell Biol 17, 350–363 (2016). https://doi.org/10.1038/nrm.2016.37
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm.2016.37
This article is cited by
-
Primase promotes the competition between transcription and replication on the same template strand resulting in DNA damage
Nature Communications (2024)
-
UV-induced G4 DNA structures recruit ZRF1 which prevents UV-induced senescence
Nature Communications (2023)
-
Genetic requirements for repair of lesions caused by single genomic ribonucleotides in S phase
Nature Communications (2023)
-
C16orf72/HAPSTR1/TAPR1 functions with BRCA1/Senataxin to modulate replication-associated R-loops and confer resistance to PARP disruption
Nature Communications (2023)
-
RNaseH2A downregulation drives inflammatory gene expression via genomic DNA fragmentation in senescent and cancer cells
Communications Biology (2022)